Abstract
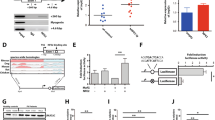
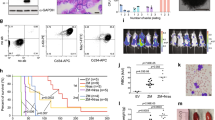
The gene encoding the transcription factor C/EBPα is mutated in 10–15% of acute myeloid leukemia (AML) patients. N-terminal CEBPA mutations cause ablation of full-length C/EBPα without affecting the expression of a shorter oncogenic isoform, termed p30. The mechanistic basis of p30-induced leukemogenesis is incompletely understood. Here, we demonstrate that the MLL1 histone-methyltransferase complex represents a critical actionable vulnerability in CEBPA-mutated AML. Oncogenic C/EBPα p30 and MLL1 show global co-localization on chromatin and p30 exhibits robust physical interaction with the MLL1 complex. CRISPR/Cas9-mediated mutagenesis of MLL1 results in proliferation arrest and myeloid differentiation in C/EBPα p30-expressing cells. In line, CEBPA-mutated hematopoietic progenitor cells are hypersensitive to pharmacological targeting of the MLL1 complex. Inhibitor treatment impairs proliferation and restores myeloid differentiation potential in mouse and human AML cells with CEBPA mutations. Finally, we identify the transcription factor GATA2 as a direct critical target of the p30-MLL1 interaction. Altogether, we show that C/EBPα p30 requires the MLL1 complex to regulate oncogenic gene expression and that CEBPA-mutated AML is hypersensitive to perturbation of the MLL1 complex. These findings identify the MLL1 complex as a potential therapeutic target in AML with CEBPA mutations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373:1136–52.
Zhang Y, Wang F, Chen X, Liu W, Fang J, Wang M, et al. Mutation profiling of 16 candidate genes in de novo acute myeloid leukemia patients. Front Med. 2018; https://doi.org/10.1007/s11684-018-0616-1.
Fasan A, Haferlach C, Alpermann T, Jeromin S, Grossmann V, Eder C, et al. The role of different genetic subtypes of CEBPA mutated AML. Leukemia. 2014;28:794–803.
Ahn J-S, Kim H-J, Kim Y-K, Lee S-S, Ahn S-Y, Jung S-H, et al. Assessment of a new genomic classification system in acute myeloid leukemia with a normal karyotype. Oncotarget. 2018;9:4961–8.
Hasemann MS, Lauridsen FKB, Waage J, Jakobsen JS, Frank AK, Schuster MB, et al. C/EBPα is required for long-term self-renewal and lineage priming of hematopoietic stem cells and for the maintenance of epigenetic configurations in multipotent progenitors. PLoS Genet. 2014;10:e1004079.
Pabst T, Mueller BU, Zhang P, Radomska HS, Narravula S, Schnittger S, et al. Dominant-negative mutations of CEBPA, encoding CCAAT/enhancer binding protein-alpha (C/EBPα), in acute myeloid leukemia. Nat Genet. 2001;27:263–70.
Zhang P, Iwasaki-Arai J, Iwasaki H, Fenyus ML, Dayaram T, Owens BM, et al. Enhancement of hematopoietic stem cell repopulating capacity and self-renewal in the absence of the transcription factor C/EBPα. Immunity. 2004;21:853–63.
Lin FT, MacDougald OA, Diehl AM, Lane MD. A 30-kDa alternative translation product of the CCAAT/enhancer binding protein alpha message: transcriptional activator lacking antimitotic activity. Proc Natl Acad Sci Usa. 1993;90:9606–10.
Pabst T, Mueller BU. Transcriptional dysregulation during myeloid transformation in AML. Oncogene. 2007;26:6829–37.
Su L, Tan Y, Lin H, Liu X, Yu L, Yang Y, et al. Mutational spectrum of acute myeloid leukemia patients with double CEBPA mutations based on next-generation sequencing and its prognostic significance. Oncotarget. 2018;9:24970–9.
Koschmieder S, Halmos B, Levantini E, Tenen DG. Dysregulation of the C/EBPα differentiation pathway in human cancer. J Clin Oncol. 2009;27:619–28.
Grebien F, Vedadi M, Getlik M, Giambruno R, Grover A, Avellino R, et al. Pharmacological targeting of the Wdr5-MLL interaction in C/EBPα N-terminal leukemia. Nat Chem Biol. 2015;11:571–8.
Guarnaccia A duPuy, Tansey WP. Moonlighting with WDR5: a cellular multitasker. J Clin Med. 2018;7:21.
Gan T, Jude CD, Zaffuto K, Ernst P. Developmentally induced Mll1 loss reveals defects in postnatal haematopoiesis. Leukemia. 2010;24:1732–41.
Ernst P, Fisher JK, Avery W, Wade S, Foy D, Korsmeyer SJ. Definitive hematopoiesis requires the mixed-lineage leukemia gene. Dev Cell. 2004;6:437–43.
Hess JL, Yu BD, Li B, Hanson R, Korsmeyer SJ. Defects in yolk sac hematopoiesis in Mll-null embryos. Blood. 1997;90:1799–806.
McMahon KA, Hiew SY-L, Hadjur S, Veiga-Fernandes H, Menzel U, Price AJ, et al. Mll has a critical role in fetal and adult hematopoietic stem cell self-renewal. Cell Stem Cell. 2007;1:338–45.
van Nuland R, Smits AH, Pallaki P, Jansen PWTC, Vermeulen M, Timmers HTM. Quantitative dissection and stoichiometry determination of the human SET1/MLL histone methyltransferase complexes. Mol Cell Biol. 2013;33:2067–77.
Dou Y, Milne TA, Ruthenburg AJ, Lee S, Lee JW, Verdine GL, et al. Regulation of MLL1 H3K4 methyltransferase activity by its core components. Nat Struct Mol Biol. 2006;13:713–9.
Cao F, Chen Y, Cierpicki T, Liu Y, Basrur V, Lei M, et al. An Ash2L/RbBP5 heterodimer stimulates the MLL1 methyltransferase activity through coordinated substrate interactions with the MLL1 SET domain. PLoS ONE. 2010;5:1–11.
Yokoyama A, Somervaille TCP, Smith KS, Rozenblatt-Rosen O, Meyerson M, Cleary ML. The menin tumor suppressor protein is an essential oncogenic cofactor for MLL-associated leukemogenesis. Cell . 2005;123:207–18.
Yokoyama A, Cleary ML. Menin critically links MLL proteins with LEDGF on cancer-associated target genes. Cancer Cell. 2008;14:36–46.
El Ashkar S, Schwaller J, Pieters T, Goossens S, Demeulemeester J, Christ F, et al. LEDGF/p75 is dispensable for hematopoiesis but essential for MLL-rearranged leukemogenesis. Blood. 2017;131:95–107.
Kirstetter P, Schuster MB, Bereshchenko O, Moore S, Dvinge H, Kurz E, et al. Modeling of C/EBPα mutant acute myeloid leukemia reveals a common expression signature of committed myeloid leukemia-initiating cells. Cancer Cell. 2008;13:299–310.
He S, Calcagno C, Lobatto ME, Robson PM, Millon A, Grembecka J. Menin-MLL inhibitors block oncogenic transformation by MLL fusion proteins in a fusion independant manner. Leukemia. 2016;28:1304–14.
Grembecka J, He S, Shi A, Purohit T, Muntean AG, Sorenson RJ, et al. Menin-MLL inhibitors reverse oncogenic activity of MLL fusion proteins in leukemia. Nat Chem Biol. 2012;8:277–84.
Shi A, Murai MJ, He S, Lund G, Hartley T, Purohit T, et al. Structural insights into inhibition of the bivalent menin-MLL interaction by small molecules in leukemia. Blood. 2012;120:4461–9.
Borkin D, He S, Miao H, Kempinska K, Pollock J, Chase J, et al. Pharmacologic inhibition of the menin-MLL interaction blocks progression of MLL leukemia in vivo. Cancer Cell. 2015;27:589–602.
Kühn MWM, Song E, Feng Z, Sinha A, Chen C, Deshpande AJ, et al. Targeting chromatin regulators inhibits leukemogenic gene expression in NPM1 mutant leukemia. Cancer Discov. 2016;6:1166–81.
Bereshchenko O, Mancini E, Moore S, Bilbao D, Månsson R, Luc S, et al. Hematopoietic stem cell expansion precedes the generation of committed myeloid leukemia-initiating cells in C/EBPα Mutant AML. Cancer Cell. 2009;16:390–400.
Zhang H, Alberich-Jorda M, Amabile G, Yang H, Staber PB, Di Ruscio A, et al. Sox4 is a key oncogenic target in C/EBPα mutant acute myeloid leukemia. Cancer Cell. 2013;24:575–88.
Nandakumar SK, Johnson K, Throm SL, Pestina TI, Neale G, Persons DA. Low-level GATA2 overexpression promotes myeloid progenitor self-renewal and blocks lymphoid differentiation in mice. Exp Hematol. 2015;43:565–77.
Luesink M, Hollink IHIM, van der Velden VH, Knops RHJN, Boezeman JBM, Haas D, et al. High GATA2 expression is a poor prognostic marker in pediatric acute myeloid leukemia. Blood. 2017;120:2064–76.
Johnson KD, Kong G, Gao X, Chang YI, Hewitt KJ, Sanalkumar R, et al. Cis-regulatory mechanisms governing stem and progenitor cell transitions. Sci Adv. 2015;1:e1500503.
Mishra BP, Zaffuto KM, Artinger EL, Org T, Mikkola HKA, Cheng C, et al. The histone methyltransferase activity of MLL1 is dispensable for hematopoiesis and leukemogenesis. Cell Rep. 2014;7:1239–47.
Lee JH, Voo KS, Skalnik DG. Identification and characterization of the DNA binding domain of CpG-binding protein. J Biol Chem. 2001;276:44669–76.
Jacobson RH, Ladurner AG, King DS, Tjian R. Structure and function of a human TAFII250 double bromodomain module. Science. 2000;288:1422–5.
Chang P-Y, Hom RA, Musselman CA, Zhu L, Kuo A, Gozani O, et al. Binding of the MLL PHD3 finger to histone H3K4me3 is required for MLL-dependent gene transcription. J Mol Biol. 2010;400:137–44.
Grembecka J, Belcher AM, Hartley T, Cierpicki T. Molecular basis of the mixed lineage leukemia-menin interaction: implications for targeting mixed lineage leukemias. J Biol Chem. 2010;285:40690–8.
Ayton PM, Cleary ML. Transformation of myeloid progenitors by MLL oncoproteins is dependent on Hoxa7 and Hoxa9. Genes Dev. 2003;17:2298–307.
Wong P, Iwasaki M, Somervaille TCP, So CWE, So CWE, Cleary ML. Meis1 is an essential and rate-limiting regulator of MLL leukemia stem cell potential. Genes Dev. 2007;21:2762–74.
Marcucci G, Maharry K, Radmacher MD, Mrózek K, Vukosavljevic T, Paschka P, et al. Prognostic significance of, and gene and microRNA expression signatures associated with, CEBPA mutations in cytogenetically normal acute myeloid leukemia with high-risk molecular features: a Cancer and Leukemia Group B Study. J Clin Oncol. 2008;26:5078–87.
Milne TA, Dou Y, Martin ME, Brock HW, Roeder RG, Hess JL. MLL associates specifically with a subset of transcriptionally active target genes. Proc Natl Acad Sci USA. 2005;102:14765–70.
Zhou W, Chung YJ, Parrilla Castellar ER, Zheng Y, Chung HJ, Bandle R, et al. Far upstream element binding protein plays a crucial role in embryonic development, hematopoiesis, and stabilizing myc expression levels. Am J Pathol. 2016;186:701–15.
Buchanan J, Tirado CA. A t(16;21)(p11; q22) in acute myeloid leukemia (AML) resulting in fusion of the FUS/TLS and ERG genes: a review of the literature. J Assoc Genet Technol. 2016;42:24–33.
Nalbant D, Youn H, Nalbant SI, Sharma S, Cobos E, Beale EG, et al. FAM20: an evolutionarily conserved family of secreted proteins expressed in hematopoietic cells. BMC Genom. 2005;6:11.
Tsai FY, Keller G, Kuo FC, Weiss M, Chen J, Rosenblatt M, et al. An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature. 1994;371:221–6.
Rodrigues NP, Janzen V, Forkert R, Dombkowski DM, Boyd AS, Orkin SH, et al. Haploinsufficiency of GATA-2 perturbs adult hematopoietic stem-cell homeostasis. Blood. 2005;106:477–84.
Rabenhorst U, Thalheimer FB, Gerlach K, Kijonka M, Böhm S, Krause DS, et al. Single-stranded DNA-binding transcriptional regulator FUBP1 is essential for fetal and adult hematopoietic stem cell self-renewal. Cell Rep. 2015;11:1847–55.
Minegishi N, Suzuki N, Yokomizo T, Pan X, Fujimoto T, Takahashi S, et al. Expression and domain-specific function of GATA-2 during differentiation of the hematopoietic precursor cells in midgestation mouse embryos. Blood. 2003;102:896–905.
Persons DA, Allay JA, Allay ER, Ashmun RA, Orlic D, Jane SM, et al. Enforced expression of the GATA-2 transcription factor blocks normal hematopoiesis. Blood. 1999;93:488–99.
Fujimaki S, Harigae H, Sugawara T, Takasawa N, Sasaki T, Kaku M. Decreased expression of transcription factor GATA-2 in haematopoietic stem cells in patients with aplastic anaemia. Br J Haematol. 2001;113:52–7.
Xu Y, Takahashi Y, Wang Y, Hama A, Nishio N, Muramatsu H, et al. Downregulation of GATA-2 and overexpression of adipogenic gene-PPARgamma in mesenchymal stem cells from patients with aplastic anemia. Exp Hematol. 2009;37:1393–9.
Zhang S-J, Ma L-Y, Huang Q-H, Li G, Gu B-W, Gao X-D, et al. Gain-of-function mutation of GATA-2 in acute myeloid transformation of chronic myeloid leukemia. Proc Natl Acad Sci USA. 2008;105:2076–81.
Vicente C, Vazquez I, Conchillo A, García-Sánchez MA, Marcotegui N, Fuster O, et al. Overexpression of GATA2 predicts an adverse prognosis for patients with acute myeloid leukemia and it is associated with distinct molecular abnormalities. Leukemia. 2012;26:550–4.
Nguyen J, Alexander T, Jiang H, Hill N, Abdullaev Z, Pack SD, et al. Melanoma in patients with GATA2 deficiency. Pigment Cell Melanoma Res. 2018;31:337–40.
Chong C-E, Venugopal P, Stokes PH, Lee YK, Brautigan PJ, Yeung DTO, et al. Differential effects on gene transcription and hematopoietic differentiation correlate with GATA2 mutant disease phenotypes. Leukemia. 2018;32:194–202.
Author contributions
L. Schmidt and F.G. designed research; L. Schmidt, E.H., L. Scheiblecker, and F.G. performed experiments and analyzed data; T.E. performed bioinformatic analysis; G.V., J.F., J.G., C.N., and P.V. provided essential material and discussion; L. Schmidt, E.H., L. Scheiblecker, and F.G. wrote the manuscript.
Support
We thank J. Zuber for murine leukemia cells and the RT3REVIN vector and J. Bigenzahn for the LentiGuide-Puro-IRES-GFP vector. NGS was performed at the VBCF NGS Unit (www.vbcf.ac.at). This work was supported by Bloodwise Specialist Programs (Grants 12010 (J.F.) and 13008 (C.N.)), by Medical Research Council Grants G0701761, G0900892 and MC_UU_12009/7 (C.N.), by an Austrian Science Fund (FWF) SFB grant F4704 (P.V.), by the National Institute of Health (NIH) grant R01 (1R01CA160467) (J.G.) and by a grant from the European Research Council under the European Union’s Horizon 2020 research and innovation program (grant agreement n° 636855/StG) (F.G.). L. Schmidt is a recipient of a DOC Fellowship of the Austrian Academy of Sciences at the Ludwig Boltzmann Institute for Cancer Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
P.V. received honoraria from Novartis, Incyte, Celgene, and Pfizer and a research grant from Incyte. J.G. receives research support from Kura Oncology, Inc and has an equity ownership in the company. Other coauthors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Schmidt, L., Heyes, E., Scheiblecker, L. et al. CEBPA-mutated leukemia is sensitive to genetic and pharmacological targeting of the MLL1 complex. Leukemia 33, 1608–1619 (2019). https://doi.org/10.1038/s41375-019-0382-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-019-0382-3
This article is cited by
-
TET2 lesions enhance the aggressiveness of CEBPA-mutant acute myeloid leukemia by rebalancing GATA2 expression
Nature Communications (2023)
-
Comprehensive assessment of differential ChIP-seq tools guides optimal algorithm selection
Genome Biology (2022)
-
Menin is necessary for long term maintenance of meningioma-1 driven leukemia
Leukemia (2021)
-
Identification of gene targets of mutant C/EBPα reveals a critical role for MSI2 in CEBPA-mutated AML
Leukemia (2021)
-
Therapeutic implications of menin inhibition in acute leukemias
Leukemia (2021)