Key Points
-
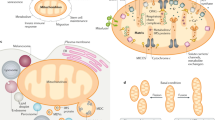
Mitochondria carry out crucial cellular functions such as energy harvesting. They possess their own genome, encoding 13 proteins, although most of the ∼1,200 mitochondrial proteins originate from the nucleus. Therefore, the nucleus and mitochondria have to constantly communicate to adjust their activities in order to ensure cellular homeostasis and adaptation to mitochondrial stress. This communication is defined as mitonuclear communication.
-
The nucleus modulates gene expression and mitochondrial function through anterograde regulation signalling. Conversely, mitochondria can elicit a retrograde response, on the basis of energetic cues, ROS or calcium signalling, that activates the expression of nuclear genes to respond and adapt to those cellular conditions.
-
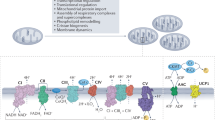
Proteostatic stress in the mitochondria can initiate many feedback responses, such as the mitochondrial unfolded protein response (UPRmt), the proteolytic stress response and the heat shock response, which directly modulate nuclear gene expression and are involved in alleviating the stress.
-
In order to induce a more general adaptation to a cellular state, mitochondrial stress can trigger the integrated stress response (ISR), which reduces cytosolic protein synthesis globally and induces the expression of cellular stress response genes.
-
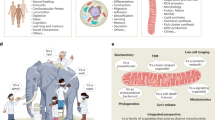
Mitochondrial stress can be signalled to other cells of the organism by extracellular cues, such as the so-called mitokines, to orchestrate the coordinated adaptation of the whole organism to stress.
-
Given their central role in cellular metabolism and in the maintenance of cellular homeostasis, mitochondria are closely involved in the ageing process. Therefore, several mitochondrial stress and mitonuclear communication pathways modulate lifespan across species.
Abstract
Mitochondria participate in crucial cellular processes such as energy harvesting and intermediate metabolism. Although mitochondria possess their own genome — a vestige of their bacterial origins and endosymbiotic evolution — most mitochondrial proteins are encoded in the nucleus. The expression of the mitochondrial proteome hence requires tight coordination between the two genomes to adapt mitochondrial function to the ever-changing cellular milieu. In this Review, we focus on the pathways that coordinate the communication between mitochondria and the nucleus during homeostasis and mitochondrial stress. These pathways include nucleus-to-mitochondria (anterograde) and mitochondria-to-nucleus (retrograde) communication, mitonuclear feedback signalling and proteostasis regulation, the integrated stress response and non-cell-autonomous communication. We discuss how mitonuclear communication safeguards cellular and organismal fitness and regulates lifespan.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Friedman, J. R. & Nunnari, J. Mitochondrial form and function. Nature 505, 335–343 (2014).
Wallace, D. C. Mitochondria, bioenergetics, and the epigenome in eukaryotic and human evolution. Cold Spring Harb. Symp. Quant. Biol. 74, 383–393 (2009).
Pagliarini, D. J. et al. A mitochondrial protein compendium elucidates complex I disease biology. Cell 134, 112–123 (2008).
Mercer, T. R. et al. The human mitochondrial transcriptome. Cell 146, 645–658 (2011).
Dhar, S. S., Ongwijitwat, S. & Wong-Riley, M. T. Nuclear respiratory factor 1 regulates all ten nuclear-encoded subunits of cytochrome c oxidase in neurons. J. Biol. Chem. 283, 3120–3129 (2008).
Virbasius, J. V. & Scarpulla, R. C. Activation of the human mitochondrial transcription factor A gene by nuclear respiratory factors: a potential regulatory link between nuclear and mitochondrial gene expression in organelle biogenesis. Proc. Natl Acad. Sci. USA 91, 1309–1313 (1994).
Gleyzer, N., Vercauteren, K. & Scarpulla, R. C. Control of mitochondrial transcription specificity factors (TFB1M and TFB2M) by nuclear respiratory factors (NRF-1 and NRF-2) and PGC-1 family coactivators. Mol. Cell. Biol. 25, 1354–1366 (2005).
Blesa, J. R., Prieto-Ruiz, J. A., Hernandez, J. M. & Hernandez-Yago, J. NRF-2 transcription factor is required for human TOMM20 gene expression. Gene 391, 198–208 (2007).
Peralta, S., Wang, X. & Moraes, C. T. Mitochondrial transcription: lessons from mouse models. Biochim. Biophys. Acta 1819, 961–969 (2012).
Richter-Dennerlein, R., Dennerlein, S. & Rehling, P. Integrating mitochondrial translation into the cellular context. Nat. Rev. Mol. Cell Biol. 16, 586–592 (2015).
Wang, Y. X. et al. Peroxisome-proliferator-activated receptor δ activates fat metabolism to prevent obesity. Cell 113, 159–170 (2003).
Tanaka, T. et al. Activation of peroxisome proliferator-activated receptor δ induces fatty acid β-oxidation in skeletal muscle and attenuates metabolic syndrome. Proc. Natl Acad. Sci. USA 100, 15924–15929 (2003).
Michalik, L. et al. International Union of Pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacol. Rev. 58, 726–741 (2006).
Dufour, C. R. et al. Genome-wide orchestration of cardiac functions by the orphan nuclear receptors ERRα and γ. Cell Metab. 5, 345–356 (2007).
Alaynick, W. A. et al. ERRγ directs and maintains the transition to oxidative metabolism in the postnatal heart. Cell Metab. 6, 13–24 (2007).
Mouchiroud, L., Eichner, L. J., Shaw, R. J. & Auwerx, J. Transcriptional coregulators: fine-tuning metabolism. Cell Metab. 20, 26–40 (2014).
Fernandez-Marcos, P. J. & Auwerx, J. Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am. J. Clin. Nutr. 93, 884S–890S (2011).
Handschin, C. & Spiegelman, B. M. Peroxisome proliferator-activated receptor γ coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr. Rev. 27, 728–735 (2006).
Yamamoto, H. et al. NCoR1 is a conserved physiological modulator of muscle mass and oxidative function. Cell 147, 827–839 (2011).
Seth, A. et al. The transcriptional corepressor RIP140 regulates oxidative metabolism in skeletal muscle. Cell Metab. 6, 236–245 (2007).
Canto, C. et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458, 1056–1060 (2009).
Canto, C. et al. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab. 11, 213–219 (2010).
Garcia-Roves, P. M., Osler, M. E., Holmstrom, M. H. & Zierath, J. R. Gain-of-function R225Q mutation in AMP-activated protein kinase γ3 subunit increases mitochondrial biogenesis in glycolytic skeletal muscle. J. Biol. Chem. 283, 35724–35734 (2008).
Wu, H. et al. Regulation of mitochondrial biogenesis in skeletal muscle by CaMK. Science 296, 349–352 (2002).
Woods, A. et al. Ca2+/calmodulin-dependent protein kinase kinase-β acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2, 21–33 (2005). References 21–25 give examples of how different kinases signal to induce mitochondrial biogenesis.
Sahin, E. et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature 470, 359–365 (2011). A description of how telomere dysfunction can initiate reprogramming of mitochondrial regulation.
Luo, X. & Kraus, W. L. On PAR with PARP: cellular stress signaling through poly(ADP-ribose) and PARP-1. Genes Dev. 26, 417–432 (2012).
Bai, P. et al. PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab. 13, 461–468 (2011).
Canto, C. et al. The NAD+ precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 15, 838–847 (2012).
Jazwinski, S. M. The retrograde response: when mitochondrial quality control is not enough. Biochim. Biophys. Acta 1833, 400–409 (2013).
Liu, Z. & Butow, R. A. Mitochondrial retrograde signaling. Annu. Rev. Genet. 40, 159–185 (2006).
Sekito, T., Thornton, J. & Butow, R. A. Mitochondria-to-nuclear signaling is regulated by the subcellular localization of the transcription factors Rtg1p and Rtg3p. Mol. Biol. Cell 11, 2103–2115 (2000).
Jazwinski, S. M. & Kriete, A. The yeast retrograde response as a model of intracellular signaling of mitochondrial dysfunction. Front. Physiol. 3, 139 (2012).
Friis, R. M. et al. Rewiring AMPK and mitochondrial retrograde signaling for metabolic control of aging and histone acetylation in respiratory-defective cells. Cell Rep. 7, 565–574 (2014).
Heeren, G. et al. The mitochondrial ribosomal protein of the large subunit, Afo1p, determines cellular longevity through mitochondrial back-signaling via TOR1. Aging 1, 622–636 (2009).
Caballero, A. et al. Absence of mitochondrial translation control proteins extends life span by activating sirtuin-dependent silencing. Mol. Cell 42, 390–400 (2011). References 34–36 give examples of energetic retrograde signals in yeast involving the Ampk, TOR and Sir2 pathways.
Curtis, R., O'Connor, G. & DiStefano, P. S. Aging networks in Caenorhabditis elegans: AMP-activated protein kinase (aak-2) links multiple aging and metabolism pathways. Aging Cell 5, 119–126 (2006).
Apfeld, J., O'Connor, G., McDonagh, T., DiStefano, P. S. & Curtis, R. The AMP-activated protein kinase AAK-2 links energy levels and insulin-like signals to lifespan in C. elegans. Genes Dev. 18, 3004–3009 (2004).
Edwards, C. B., Copes, N., Brito, A. G., Canfield, J. & Bradshaw, P. C. Malate and fumarate extend lifespan in Caenorhabditis elegans. PLoS ONE 8, e58345 (2013).
Gallo, M., Park, D. & Riddle, D. L. Increased longevity of some C. elegans mitochondrial mutants explained by activation of an alternative energy-producing pathway. Mech. Ageing Dev. 132, 515–518 (2011).
Chin, R. M. et al. The metabolite α-ketoglutarate extends lifespan by inhibiting ATP synthase and TOR. Nature 510, 397–401 (2014).
Egan, D. F. et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science 331, 456–461 (2011).
Lerner, C. et al. Reduced mammalian target of rapamycin activity facilitates mitochondrial retrograde signaling and increases life span in normal human fibroblasts. Aging Cell 12, 966–977 (2013).
Rizzuto, R., De Stefani, D., Raffaello, A. & Mammucari, C. Mitochondria as sensors and regulators of calcium signalling. Nat. Rev. Mol. Cell Biol. 13, 566–578 (2012).
Arnould, T. et al. CREB activation induced by mitochondrial dysfunction is a new signaling pathway that impairs cell proliferation. EMBO J. 21, 53–63 (2002).
Luo, Y., Bond, J. D. & Ingram, V. M. Compromised mitochondrial function leads to increased cytosolic calcium and to activation of MAP kinases. Proc. Natl Acad. Sci. USA 94, 9705–9710 (1997).
Amuthan, G. et al. Mitochondrial stress-induced calcium signaling, phenotypic changes and invasive behavior in human lung carcinoma A549 cells. Oncogene 21, 7839–7849 (2002).
Srinivasan, S. et al. Disruption of cytochrome c oxidase function induces the Warburg effect and metabolic reprogramming. Oncogene http://dx.doi.org/10.1038/onc.2015.227, (2015).
Biswas, G., Anandatheerthavarada, H. K., Zaidi, M. & Avadhani, N. G. Mitochondria to nucleus stress signaling: a distinctive mechanism of NFκB/Rel activation through calcineurin-mediated inactivation of IκBβ. J. Cell Biol. 161, 507–519 (2003).
Biswas, G. et al. Retrograde Ca2+ signaling in C2C12 skeletal myocytes in response to mitochondrial genetic and metabolic stress: a novel mode of inter-organelle crosstalk. EMBO J. 18, 522–533 (1999).
Formentini, L., Sanchez-Arago, M., Sanchez-Cenizo, L. & Cuezva, J. M. The mitochondrial ATPase inhibitory factor 1 triggers a ROS-mediated retrograde prosurvival and proliferative response. Mol. Cell 45, 731–742 (2012).
Amuthan, G. et al. Mitochondria-to-nucleus stress signaling induces phenotypic changes, tumor progression and cell invasion. EMBO J. 20, 1910–1920 (2001).
Lim, J. H. et al. Mitochondrial dysfunction induces aberrant insulin signalling and glucose utilisation in murine C2C12 myotube cells. Diabetologia 49, 1924–1936 (2006).
Guha, M., Fang, J. K., Monks, R., Birnbaum, M. J. & Avadhani, N. G. Activation of Akt is essential for the propagation of mitochondrial respiratory stress signaling and activation of the transcriptional coactivator heterogeneous ribonucleoprotein A2. Mol. Biol. Cell 21, 3578–3589 (2010).
Schieber, M. & Chandel, N. S. ROS function in redox signaling and oxidative stress. Curr. Biol. 24, R453–R462 (2014).
Shadel, G. S. & Horvath, T. L. Mitochondrial ROS signaling in organismal homeostasis. Cell 163, 560–569 (2015).
Miyadera, H. et al. Altered quinone biosynthesis in the long-lived clk-1 mutants of Caenorhabditis elegans. J. Biol. Chem. 276, 7713–7716 (2001).
Lee, S. J., Hwang, A. B. & Kenyon, C. Inhibition of respiration extends C. elegans life span via reactive oxygen species that increase HIF-1 activity. Curr. Biol. 20, 2131–2136 (2010). Identification of a signalling mechanism involving ROS in lifespan extension following mild respiration inhibition.
Inoue, H. et al. The C. elegans p38 MAPK pathway regulates nuclear localization of the transcription factor SKN-1 in oxidative stress response. Genes Dev. 19, 2278–2283 (2005).
Zarse, K. et al. Impaired insulin/IGF1 signaling extends life span by promoting mitochondrial l-proline catabolism to induce a transient ROS signal. Cell Metab. 15, 451–465 (2012).
Schmeisser, S. et al. Mitochondrial hormesis links low-dose arsenite exposure to lifespan extension. Aging Cell 12, 508–517 (2013).
Monaghan, R. M. et al. A nuclear role for the respiratory enzyme CLK-1 in regulating mitochondrial stress responses and longevity. Nat. Cell Biol. 17, 782–792 (2015). Identification of a novel communication mechanism involving the nuclear translocation of an isoform of CLK-1, which regulates stress resistance and lifespan.
Owusu-Ansah, E., Yavari, A., Mandal, S. & Banerjee, U. Distinct mitochondrial retrograde signals control the G1–S cell cycle checkpoint. Nat. Genet. 40, 356–361 (2008).
Owusu-Ansah, E., Song, W. & Perrimon, N. Muscle mitohormesis promotes longevity via systemic repression of insulin signaling. Cell 155, 699–712 (2013). A description of non-cell-autonomous-mediated lifespan extension through ROS, the UPRmt and insulin signalling following mitochondrial perturbation in D. melanogaster muscles.
Lu, W. et al. ZNF143 transcription factor mediates cell survival through upregulation of the GPX1 activity in the mitochondrial respiratory dysfunction. Cell Death Dis. 3, e422 (2012).
Chen, X. L. & Kunsch, C. Induction of cytoprotective genes through Nrf2/antioxidant response element pathway: a new therapeutic approach for the treatment of inflammatory diseases. Curr. Pharm. Des. 10, 879–891 (2004).
Kops, G. J. et al. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature 419, 316–321 (2002).
Tan, W. Q., Wang, K., Lv, D. Y. & Li, P. F. Foxo3a inhibits cardiomyocyte hypertrophy through transactivating catalase. J. Biol. Chem. 283, 29730–29739 (2008).
Nguyen, T., Nioi, P. & Pickett, C. B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 284, 13291–13295 (2009).
Chae, S. et al. A systems approach for decoding mitochondrial retrograde signaling pathways. Sci. Signal. 6, rs4 (2013).
Acin-Perez, R. et al. ROS-triggered phosphorylation of complex II by Fgr kinase regulates cellular adaptation to fuel use. Cell Metab. 19, 1020–1033 (2014).
Shi, S. Y. et al. DJ-1 links muscle ROS production with metabolic reprogramming and systemic energy homeostasis in mice. Nat. Commun. 6, 7415 (2015).
Quiros, P. M., Langer, T. & Lopez-Otin, C. New roles for mitochondrial proteases in health, ageing and disease. Nat. Rev. Mol. Cell Biol. 16, 345–359 (2015).
Jovaisaite, V., Mouchiroud, L. & Auwerx, J. The mitochondrial unfolded protein response, a conserved stress response pathway with implications in health and disease. J. Exp. Biol. 217, 137–143 (2014).
Houtkooper, R. H. et al. Mitonuclear protein imbalance as a conserved longevity mechanism. Nature 497, 451–457 (2013). Establishment of the concept of mitonuclear imbalance to explain the identification of Mrps5 as a mouse longevity gene and the pro-longevity effect of mitochondrial translation inhibition.
Durieux, J., Wolff, S. & Dillin, A. The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell 144, 79–91 (2011). The first description of non-cell-autonomous-mediated longevity following mitochondrial stress.
Mouchiroud, L. et al. The NAD+/sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling. Cell 154, 430–441 (2013).
Pirinen, E. et al. Pharmacological inhibition of poly(ADP-ribose) polymerases improves fitness and mitochondrial function in skeletal muscle. Cell Metab. 19, 1034–1041 (2014).
Gariani, K. et al. Eliciting the mitochondrial unfolded protein response via NAD repletion reverses fatty liver disease. Hepatology http://dx.doi.org/10.1002/hep.28245, (2015). References 21, 22, 28–29 and 77–79 describe mechanisms of mitochondrial regulation involving NAD+ and SIRT1.
Haynes, C. M., Yang, Y., Blais, S. P., Neubert, T. A. & Ron, D. The matrix peptide exporter HAF-1 signals a mitochondrial UPR by activating the transcription factor ZC376.7 in C. elegans. Mol. Cell 37, 529–540 (2010).
Nargund, A. M., Pellegrino, M. W., Fiorese, C. J., Baker, B. M. & Haynes, C. M. Mitochondrial import efficiency of ATFS-1 regulates mitochondrial UPR activation. Science 337, 587–590 (2012).
Benedetti, C., Haynes, C. M., Yang, Y., Harding, H. P. & Ron, D. Ubiquitin-like protein 5 positively regulates chaperone gene expression in the mitochondrial unfolded protein response. Genetics 174, 229–239 (2006).
Haynes, C. M., Petrova, K., Benedetti, C., Yang, Y. & Ron, D. ClpP mediates activation of a mitochondrial unfolded protein response in C. elegans. Dev. Cell 13, 467–480 (2007).
Nargund, A. M., Fiorese, C. J., Pellegrino, M. W., Deng, P. & Haynes, C. M. Mitochondrial and nuclear accumulation of the transcription factor ATFS-1 promotes OXPHOS recovery during the UPRmt. Mol. Cell 58, 123–133 (2015). References 80–84 unravel the molecular mechanisms of UPRmt regulation in worms.
Pimenta de Castro, I. et al. Genetic analysis of mitochondrial protein misfolding in Drosophila melanogaster. Cell Death Differ. 19, 1308–1316 (2012).
Horibe, T. & Hoogenraad, N. J. The chop gene contains an element for the positive regulation of the mitochondrial unfolded protein response. PLoS ONE 2, e835 (2007).
Zhao, Q. et al. A mitochondrial specific stress response in mammalian cells. EMBO J. 21, 4411–4419 (2002). The first description of the UPRmt.
Aldridge, J. E., Horibe, T. & Hoogenraad, N. J. Discovery of genes activated by the mitochondrial unfolded protein response (mtUPR) and cognate promoter elements. PLoS ONE 2, e874 (2007).
Kohler, F., Muller-Rischart, A. K., Conradt, B. & Rolland, S. G. The loss of LRPPRC function induces the mitochondrial unfolded protein response. Aging 7, 701–717 (2015).
Papa, L. & Germain, D. SirT3 regulates the mitochondrial unfolded protein response. Mol. Cell. Biol. 34, 699–710 (2014).
Al-Furoukh, N. et al. ClpX stimulates the mitochondrial unfolded protein response (UPRmt) in mammalian cells. Biochim. Biophys. Acta 1853, 2580–2591 (2015).
Siegelin, M. D. et al. Exploiting the mitochondrial unfolded protein response for cancer therapy in mice and human cells. J. Clin. Invest. 121, 1349–1360 (2011).
Jin, S. M. & Youle, R. J. The accumulation of misfolded proteins in the mitochondrial matrix is sensed by PINK1 to induce PARK2/Parkin-mediated mitophagy of polarized mitochondria. Autophagy 9, 1750–1757 (2013).
Dogan, S. A. et al. Tissue-specific loss of DARS2 activates stress responses independently of respiratory chain deficiency in the heart. Cell Metab. 19, 458–469 (2014).
Song, M., Mihara, K., Chen, Y., Scorrano, L. & Dorn, G. W. 2nd. Mitochondrial fission and fusion factors reciprocally orchestrate mitophagic culling in mouse hearts and cultured fibroblasts. Cell Metab. 21, 273–285 (2015).
Khan, N. A. et al. Effective treatment of mitochondrial myopathy by nicotinamide riboside, a vitamin B3 . EMBO Mol. Med. 6, 721–731 (2014).
Ryu, D. et al. A SIRT7-dependent acetylation switch of GABPβ1 controls mitochondrial function. Cell Metab. 20, 856–869 (2014).
Mohrin, M. et al. A mitochondrial UPR-mediated metabolic checkpoint regulates hematopoietic stem cell aging. Science 347, 1374–1377 (2015).
Wu, Y. et al. Multilayered genetic and omics dissection of mitochondrial activity in a mouse reference population. Cell 158, 1415–1430 (2014).
Papa, L. & Germain, D. Estrogen receptor mediates a distinct mitochondrial unfolded protein response. J. Cell Sci. 124, 1396–1402 (2011).
Bahat, A. et al. Transcriptional activation of LON gene by a new form of mitochondrial stress: a role for the nuclear respiratory factor 2 in StAR overload response (SOR). Mol. Cell Endocrinol. 408, 62–72 (2015).
Bahat, A. et al. StAR enhances transcription of genes encoding the mitochondrial proteases involved in its own degradation. Mol. Endocrinol. 28, 208–224 (2014).
Akerfelt, M., Morimoto, R. I. & Sistonen, L. Heat shock factors: integrators of cell stress, development and lifespan. Nat. Rev. Mol. Cell Biol. 11, 545–555 (2010).
Tan, K. et al. Mitochondrial SSBP1 protects cells from proteotoxic stresses by potentiating stress-induced HSF1 transcriptional activity. Nat. Commun. 6, 6580 (2015).
Harding, H. P. et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell 6, 1099–1108 (2000).
Harding, H. P. et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell 11, 619–633 (2003).
Donnelly, N., Gorman, A. M., Gupta, S. & Samali, A. The eIF2α kinases: their structures and functions. Cell. Mol. Life Sci. 70, 3493–3511 (2013).
Palam, L. R., Baird, T. D. & Wek, R. C. Phosphorylation of eIF2 facilitates ribosomal bypass of an inhibitory upstream ORF to enhance CHOP translation. J. Biol. Chem. 286, 10939–10949 (2011).
Novoa, I., Zeng, H., Harding, H. P. & Ron, D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2α. J. Cell Biol. 153, 1011–1022 (2001).
Ohoka, N., Yoshii, S., Hattori, T., Onozaki, K. & Hayashi, H. TRB3, a novel ER stress-inducible gene, is induced via ATF4–CHOP pathway and is involved in cell death. EMBO J. 24, 1243–1255 (2005).
Baker, B. M., Nargund, A. M., Sun, T. & Haynes, C. M. Protective coupling of mitochondrial function and protein synthesis via the eIF2α kinase GCN-2. PLoS Genet. 8, e1002760 (2012).
Rainbolt, T. K., Atanassova, N., Genereux, J. C. & Wiseman, R. L. Stress-regulated translational attenuation adapts mitochondrial protein import through Tim17A degradation. Cell Metab. 18, 908–919 (2013).
Michel, S., Canonne, M., Arnould, T. & Renard, P. Inhibition of mitochondrial genome expression triggers the activation of CHOP-10 by a cell signaling dependent on the integrated stress response but not the mitochondrial unfolded protein response. Mitochondrion 21, 58–68 (2015).
Moullan, N. et al. Tetracyclines disturb mitochondrial function across eukaryotic models: a call for caution in biomedical research. Cell Rep. 10, 1681–1691 (2015).
Bruning, A., Brem, G. J., Vogel, M. & Mylonas, I. Tetracyclines cause cell stress-dependent ATF4 activation and mTOR inhibition. Exp. Cell Res. 320, 281–289 (2014).
Moisoi, N. et al. Mitochondrial dysfunction triggered by loss of HtrA2 results in the activation of a brain-specific transcriptional stress response. Cell Death Differ. 16, 449–464 (2009).
Evstafieva, A. G. et al. A sustained deficiency of mitochondrial respiratory complex III induces an apoptotic cell death through the p53-mediated inhibition of pro-survival activities of the activating transcription factor 4. Cell Death Dis. 5, e1511 (2014).
Martinez-Reyes, I., Sanchez-Arago, M. & Cuezva, J. M. AMPK and GCN2–ATF4 signal the repression of mitochondria in colon cancer cells. Biochem. J. 444, 249–259 (2012).
Silva, J. M., Wong, A., Carelli, V. & Cortopassi, G. A. Inhibition of mitochondrial function induces an integrated stress response in oligodendroglia. Neurobiol. Dis. 34, 357–365 (2009).
Viader, A. et al. Aberrant Schwann cell lipid metabolism linked to mitochondrial deficits leads to axon degeneration and neuropathy. Neuron 77, 886–898 (2013).
Wang, X. & Chen, X. J. A cytosolic network suppressing mitochondria-mediated proteostatic stress and cell death. Nature 524, 481–484 (2015).
Wrobel, L. et al. Mistargeted mitochondrial proteins activate a proteostatic response in the cytosol. Nature 524, 485–488 (2015). References 121 and 122 identify a cytosolic stress response activated by mitochondrial stress.
Copeland, J. M. et al. Extension of Drosophila life span by RNAi of the mitochondrial respiratory chain. Curr. Biol. 19, 1591–1598 (2009).
Lee, C. et al. The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metab. 21, 443–454 (2015).
Lee, C., Yen, K. & Cohen, P. Humanin: a harbinger of mitochondrial-derived peptides? Trends Endocrinol. Metab. 24, 222–228 (2013).
Hashimoto, Y. et al. A rescue factor abolishing neuronal cell death by a wide spectrum of familial Alzheimer's disease genes and Aβ. Proc. Natl Acad. Sci. USA 98, 6336–6341 (2001).
Chau, M. D., Gao, J., Yang, Q., Wu, Z. & Gromada, J. Fibroblast growth factor 21 regulates energy metabolism by activating the AMPK–SIRT1–PGC-1α pathway. Proc. Natl Acad. Sci. USA 107, 12553–12558 (2010).
Tyynismaa, H. et al. Mitochondrial myopathy induces a starvation-like response. Hum. Mol. Genet. 19, 3948–3958 (2010).
Suomalainen, A. et al. FGF-21 as a biomarker for muscle-manifesting mitochondrial respiratory chain deficiencies: a diagnostic study. Lancet Neurol. 10, 806–818 (2011).
Kim, K. H. et al. Autophagy deficiency leads to protection from obesity and insulin resistance by inducing Fgf21 as a mitokine. Nat. Med. 19, 83–92 (2013). References 124 and 128–130 report examples of non-cell-autonomous signalling of mitochondrial stress in mammals.
Lopez-Otin, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217 (2013).
Nunnari, J. & Suomalainen, A. Mitochondria: in sickness and in health. Cell 148, 1145–1159 (2012).
Feng, J., Bussiere, F. & Hekimi, S. Mitochondrial electron transport is a key determinant of life span in Caenorhabditis elegans. Dev. Cell 1, 633–644 (2001).
Wong, A., Boutis, P. & Hekimi, S. Mutations in the clk-1 gene of Caenorhabditis elegans affect developmental and behavioral timing. Genetics 139, 1247–1259 (1995).
Yang, W. & Hekimi, S. A mitochondrial superoxide signal triggers increased longevity in Caenorhabditis elegans. PLoS Biol. 8, e1000556 (2010).
Kirchman, P. A., Kim, S., Lai, C. Y. & Jazwinski, S. M. Interorganelle signaling is a determinant of longevity in Saccharomyces cerevisiae. Genetics 152, 179–190 (1999).
Kaeberlein, M. et al. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science 310, 1193–1196 (2005).
Hwang, A. B. et al. Feedback regulation via AMPK and HIF-1 mediates ROS-dependent longevity in Caenorhabditis elegans. Proc. Natl Acad. Sci. USA 111, E4458–E4467 (2014).
Baruah, A. et al. CEP-1, the Caenorhabditis elegans p53 homolog, mediates opposing longevity outcomes in mitochondrial electron transport chain mutants. PLoS Genet. 10, e1004097 (2014).
Walter, L., Baruah, A., Chang, H. W., Pace, H. M. & Lee, S. S. The homeobox protein CEH-23 mediates prolonged longevity in response to impaired mitochondrial electron transport chain in C. elegans. PLoS Biol. 9, e1001084 (2011).
Palikaras, K., Lionaki, E. & Tavernarakis, N. Coordination of mitophagy and mitochondrial biogenesis during ageing in C. elegans. Nature 521, 525–528 (2015). A description of how mitochondrial biogenesis and mitophagy are coordinately regulated to determine lifespan in C. elegans.
Ristow, M. Unraveling the truth about antioxidants: mitohormesis explains ROS-induced health benefits. Nat. Med. 20, 709–711 (2014).
Zhang, Y., Shao, Z., Zhai, Z., Shen, C. & Powell-Coffman, J. A. The HIF-1 hypoxia-inducible factor modulates lifespan in C. elegans. PLoS ONE 4, e6348 (2009).
Heidler, T., Hartwig, K., Daniel, H. & Wenzel, U. Caenorhabditis elegans lifespan extension caused by treatment with an orally active ROS-generator is dependent on DAF-16 and SIR-2.1. Biogerontology 11, 183–195 (2010).
Lee, H. et al. The Caenorhabditis elegans AMP-activated protein kinase AAK-2 is phosphorylated by LKB1 and is required for resistance to oxidative stress and for normal motility and foraging behavior. J. Biol. Chem. 283, 14988–14993 (2008).
Schaar, C. E. et al. Mitochondrial and cytoplasmic ROS have opposing effects on lifespan. PLoS Genet. 11, e1004972 (2015).
Liu, X. et al. Evolutionary conservation of the clk-1-dependent mechanism of longevity: loss of mclk1 increases cellular fitness and lifespan in mice. Genes Dev. 19, 2424–2434 (2005).
Lapointe, J. & Hekimi, S. Early mitochondrial dysfunction in long-lived Mclk1+/− mice. J. Biol. Chem. 283, 26217–26227 (2008).
Dell'agnello, C. et al. Increased longevity and refractoriness to Ca2+-dependent neurodegeneration in Surf1 knockout mice. Hum. Mol. Genet. 16, 431–444 (2007).
Zordan, M. A. et al. Post-transcriptional silencing and functional characterization of the Drosophila melanogaster homolog of human Surf1. Genetics 172, 229–241 (2006).
Pulliam, D. A. et al. Complex IV-deficient Surf1−/− mice initiate mitochondrial stress responses. Biochem. J. 462, 359–371 (2014).
Caldeira da Silva, C. C., Cerqueira, F. M., Barbosa, L. F., Medeiros, M. H. & Kowaltowski, A. J. Mild mitochondrial uncoupling in mice affects energy metabolism, redox balance and longevity. Aging Cell 7, 552–560 (2008). References 147–152 give some examples of mitochondrial stress affecting health and lifespan in mammals.
Kim, H. J., Morrow, G., Westwood, J. T., Michaud, S. & Tanguay, R. M. Gene expression profiling implicates OXPHOS complexes in lifespan extension of flies over-expressing a small mitochondrial chaperone, Hsp22. Exp. Gerontol. 45, 611–620 (2010).
Andreux, P. A., Houtkooper, R. H. & Auwerx, J. Pharmacological approaches to restore mitochondrial function. Nat. Rev. Drug Discov. 12, 465–483 (2013).
Williams, E. G. & Auwerx, J. The convergence of systems and reductionist approaches in complex trait analysis. Cell 162, 23–32 (2015).
Yun, J. & Finkel, T. Mitohormesis. Cell Metab. 19, 757–766 (2014).
Dietrich, A., Wallet, C., Iqbal, R. K., Gualberto, J. M. & Lotfi, F. Organellar non-coding RNAs: emerging regulation mechanisms. Biochimie 117, 48–62 (2015).
Sugiura, A., McLelland, G. L., Fon, E. A. & McBride, H. M. A new pathway for mitochondrial quality control: mitochondrial-derived vesicles. EMBO J. 33, 2142–2156 (2014).
Arnoult, D., Soares, F., Tattoli, I. & Girardin, S. E. Mitochondria in innate immunity. EMBO Rep. 12, 901–910 (2011).
Liu, Y., Samuel, B. S., Breen, P. C. & Ruvkun, G. Caenorhabditis elegans pathways that surveil and defend mitochondria. Nature 508, 406–410 (2014).
Pellegrino, M. W. et al. Mitochondrial UPR-regulated innate immunity provides resistance to pathogen infection. Nature 516, 414–417 (2014).
West, A. P. et al. Mitochondrial DNA stress primes the antiviral innate immune response. Nature 520, 553–557 (2015).
West, A. P. et al. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature 472, 476–480 (2011).
Wang, D., Malo, D. & Hekimi, S. Elevated mitochondrial reactive oxygen species generation affects the immune response via hypoxia-inducible factor-1α in long-lived Mclk1+/− mouse mutants. J. Immunol. 184, 582–590 (2010).
Wang, D. et al. An enhanced immune response of Mclk1+/− mutant mice is associated with partial protection from fibrosis, cancer and the development of biomarkers of aging. PLoS ONE 7, e49606 (2012).
Manzanillo, P. S. et al. The ubiquitin ligase parkin mediates resistance to intracellular pathogens. Nature 501, 512–516 (2013).
Baixauli, F. et al. Mitochondrial respiration controls lysosomal function during inflammatory T cell responses. Cell Metab. 22, 485–498 (2015). References 160–167 report several examples of crosstalk between mitochondrial stress signalling and immunity.
Williams, D. S., Cash, A., Hamadani, L. & Diemer, T. Oxaloacetate supplementation increases lifespan in Caenorhabditis elegans through an AMPK/FOXO-dependent pathway. Aging Cell 8, 765–768 (2009).
Dillin, A. et al. Rates of behavior and aging specified by mitochondrial function during development. Science 298, 2398–2401 (2002). A description of the longevity phenotypes of C. elegans ETC mutants.
Yang, W. & Hekimi, S. Two modes of mitochondrial dysfunction lead independently to lifespan extension in Caenorhabditis elegans. Aging Cell 9, 433–447 (2010).
Liu, J. et al. Drosophila sbo regulates lifespan through its function in the synthesis of coenzyme Q in vivo. J. Genet. Genom. 38, 225–234 (2011).
Lemire, B. D., Behrendt, M., DeCorby, A. & Gaskova, D. C. elegans longevity pathways converge to decrease mitochondrial membrane potential. Mech. Ageing Dev. 130, 461–465 (2009).
Acknowledgements
The authors thank all members of the Auwerx laboratory for their helpful comments on the manuscript and apologize for the omission of relevant work owing to space constraints. P.M.Q. is supported by a long-term fellowship from the European Molecular Biology Organization (EMBO; ALTF 480–2014). J.A. is the Nestlé Chair in Energy Metabolism, and the Auwerx laboratory is supported by grants from the École Polytechnique Fédérale de Lausanne, the Swiss National Science Foundation (31003A-140780), the AgingX programme of the Swiss Initiative for Systems Biology (51RTP0-151019), the Krebsforschung Schweiz/Swiss Cancer League (KFS-3082-02-2013) and the US National Institutes of Health (NIH; R01AG043930).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Endosymbionts
-
Organisms that live within another organism in a symbiotic relationship.
- Electron transport chain
-
(ETC). A group of protein complexes that pass electrons from one to another via redox reactions coupled with the transfer of protons across the inner mitochondrial membrane, creating a proton gradient that drives ATP synthesis.
- Intermediate metabolism
-
The intermediate steps by which nutrient molecules or foodstuffs are metabolized intracellularly.
- Calcium buffering
-
A mechanism that regulates calcium ion (Ca2+) concentrations within the cytoplasm.
- Oxidative phosphorylation
-
(OXPHOS). A biochemical pathway within mitochondria that generates ATP through the oxidation of nutrients.
- Nuclear receptors
-
Ligand-dependent transcription factors that are characterized by a central DNA-binding domain and a carboxy-terminal ligand-binding domain.
- Metabolic reprogramming
-
The set of changes in cellular metabolism that allow quiescent cells to proliferate; it is considered to be a hallmark of cancer.
- Reactive oxygen species
-
(ROS). Reactive molecules generated in cells by the reduction of oxygen with a single electron (superoxide), two electrons (hydrogen peroxide) or three electrons (hydroxyl radical).
- Anaplerotic reactions
-
Reactions that replenish intermediates of metabolic pathways.
- Glyoxylate cycle
-
An anabolic pathway, and a variation of the tricarboxylic acid cycle, that converts two acetyl-CoA molecules to dicarboxylic acid (succinate); this cycle is not present in mammals.
- Mitophagy
-
A selective form of autophagy that is responsible for the elimination of defective mitochondria.
- Ionophores
-
Molecules that bind to ions and allow their passage across a membrane or a lipophilic phase.
- Mitochondrial membrane potential
-
An electrochemical potential formed by chemiosmosis that results from the proton gradient generated across the inner mitochondrial membrane by the mitochondrial respiratory chain.
- Mitochondria fuel switching
-
Adaptations in mitochondrial function and electron transport chain organization as a result of changes in the availability of the fuel source.
- Mitochondrial uncoupling
-
The dissociation of mitochondrial respiration from ATP generation, characterized by increased permeability of the inner mitochondrial membrane to protons and subsequent dissipation of mitochondrial membrane potential.
- Mitonuclear imbalance
-
Mitonuclear imbalance occurs when oxidative phosphorylation subunits encoded by both mitochondrial and nuclear DNA fail to assemble together in precise stoichiometric ratios to ensure proper mitochondrial function, owing to an increase or decrease of subunits from one of the origins.
- Mitohormesis
-
A process in which low levels of mitochondrial stress cause a protective cellular response that reduces susceptibility to disease and potentially promotes longevity.
- Mitofusins
-
High-molecular-mass GTPases that are essential for mitochondrial fusion.
- Mouse BXD genetic reference population
-
A family of recombinant inbred mouse strains, which is currently the largest and best-characterized mouse genetic reference population, composed of ∼160 lines.
- Mitokines
-
Signals released from a cell or tissue in response to mitochondrial stress to modulate cellular or organismal homeostasis and longevity.
- Ketogenesis
-
A metabolic pathway in the liver that generates ketones using acetyl-CoA, which provides an important fuel source for the brain and other tissues during long-term fasting.
- Rho0 cells
-
Eukaryotic cells that lack mitochondrial DNA.
- Formyl peptides
-
Small peptides in mitochondria and bacteria with a formylated amino-terminal Met and, usually, a hydrophobic amino acid at the carboxy-terminal.
Rights and permissions
About this article
Cite this article
Quirós, P., Mottis, A. & Auwerx, J. Mitonuclear communication in homeostasis and stress. Nat Rev Mol Cell Biol 17, 213–226 (2016). https://doi.org/10.1038/nrm.2016.23
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm.2016.23
This article is cited by
-
CTRP3 alleviates mitochondrial dysfunction and oxidative stress injury in pathological cardiac hypertrophy by activating UPRmt via the SIRT1/ATF5 axis
Cell Death Discovery (2024)
-
Protein phosphatase 1 regulatory subunit 15 A promotes translation initiation and induces G2M phase arrest during cuproptosis in cancers
Cell Death & Disease (2024)
-
Mitochondrial stress activates YAP/TAZ through RhoA oxidation to promote liver injury
Cell Death & Disease (2024)
-
Mitochondrial homeostasis: shaping health and disease
Current Medicine (2024)
-
Emerging roles of mitochondrial functions and epigenetic changes in the modulation of stem cell fate
Cellular and Molecular Life Sciences (2024)