Key Points
-
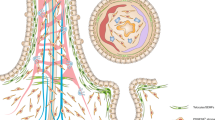
The epithelial lining of the intestine renews itself more rapidly than any other tissue in the vertebrate body, replacing the entire population of differentiated cells that cover the intestinal villi every few days.
-
Stem cells and their transit-amplifying progeny reside in intervillus pockets (in the fetus or neonate) or crypts of Lieberkühn (in the adult), and give rise to four classes of non-dividing differentiated cells — three secretory and one absorptive.
-
Transit-amplifying cells in the crypts probably become committed as secretory or absorptive progenitors several cycles before they stop dividing. The secretory progenitors express mouse atonal homologue 1 (Math1), and no secretory cells are produced when Math1 is defective.
-
Epithelial cells in each stem-cell region (crypt or pocket) produce a hedgehog signal that acts on the mesenchyme, evoking expression of bone morphogenetic protein (BMP). BMP acts back on the epithelium to inhibit formation of ectopic stem-cell regions.
-
The stem-cell regions of epithelium maintain themselves by canonical Wnt signalling, apparently through an auto-activating feedback loop.
-
When the canonical Wnt signalling pathway is blocked, proliferation fails and secretory cells are lacking; when the pathway is overactivated, proliferation is excessive and tumours develop, containing a mixture of cell types (adenomatous polyps).
-
The proliferative epithelial cells in the crypts also interact with one another by Notch signalling, mediating lateral inhibition. When the Notch signalling pathway is blocked, proliferation fails and all the crypt cells become secretory; when the pathway is overactivated, proliferation continues but no secretory cells are produced.
-
Wnt and Notch signals collaborate to maintain intestinal stem cells: neither pathway on its own is sufficient. But Wnt pathway activation can induce expression of Notch pathway components and so produce the collaborative effect.
-
Wnt signalling also regulates the expression of Eph/ephrins. Eph/ephrin signalling in turn controls the migratory behaviour that keeps proliferative and differentiated cells segregated along the crypt–villus axis.
-
Wnt and Notch signals collaborate in a remarkably similar way to maintain stem cells in the haemopoietic system and the CNS. But in some other tissues where both pathways are crucial, such as the epidermis, the rules of stem-cell maintenance are different.
Abstract
The lining of the intestine is renewed at an extraordinary rate, outpacing all other tissues in the vertebrate body. The renewal process is neatly organized in space, so that the whole production line, from the ever-youthful stem cells to their dying, terminally differentiated progeny, is laid out to view in histological sections. A flurry of recent papers has clarified the key regulatory signals and brought us to the point where we can begin to give a coherent account, for at least one tissue, of how these signals collaborate to organize the architecture and behaviour of a stem-cell system.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Goodlad, R. A. & Wright, N. A. The effects of starvation and refeeding on intestinal cell proliferation in the mouse. Virchows Arch. B Cell Pathol. 45, 63–73 (1984).
Wright, N. & Alison, M. The Biology of Epithelial Cell Populations (Clarendon, Oxford, 1984).
Cheng, H. & Leblond, C. P. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am. J. Anat. 141, 537–561 (1974).
Leedham, S. J., Brittan, M., McDonald, S. A. & Wright, N. A. Intestinal stem cells. J. Cell. Mol. Med. 9, 11–24 (2005).
Park, H. S., Goodlad, R. A. & Wright, N. A. Crypt fission in the small intestine and colon. A mechanism for the emergence of G6PD locus-mutated crypts after treatment with mutagens. Am. J. Pathol. 147, 1416–1427 (1995).
Bjerknes, M. & Cheng, H. Clonal analysis of mouse intestinal epithelial progenitors. Gastroenterology 116, 7–14 (1999). Using chemical mutagenesis to create marked clones, this paper shows that the intestinal epithelium contains not only pluripotent progenitors and stem cells, but also dividing progenitors that are destined to produce large clones of a single cell type.
Wong, M. H., Saam, J. R., Stappenbeck, T. S., Rexer, C. H. & Gordon, J. I. Genetic mosaic analysis based on Cre recombinase and navigated laser capture microdissection. Proc. Natl Acad. Sci. USA 97, 12601–12606 (2000).
Potten, C. S. Stem cells in gastrointestinal epithelium: numbers, characteristics and death. Philos. Trans. R. Soc. Lond. B 353, 821–830 (1998).
Kedinger, M. et al. Fetal gut mesenchyme induces differentiation of cultured intestinal endodermal and crypt cells. Dev. Biol. 113, 474–483 (1986).
Madison, B. B. et al. Epithelial hedgehog signals pattern the intestinal crypt–villus axis. Development 132, 279–289 (2005). Shows that the intestinal epithelium sends a hedgehog signal to the mesenchyme, and that this signal is required, through a secondary effect of the mesenchyme on the epithelium, to restrict the sites of activation of the canonical Wnt pathway and limit the development of the proliferative (crypt-like) epithelium.
Apelqvist, A., Ahlgren, U. & Edlund, H. Sonic hedgehog directs specialised mesoderm differentiation in the intestine and pancreas. Curr. Biol. 7, 801–804 (1997).
Sukegawa, A. et al. The concentric structure of the developing gut is regulated by Sonic hedgehog derived from endodermal epithelium. Development 127, 1971–1980 (2000).
Wang, L. C. et al. Disruption of hedgehog signaling reveals a novel role in intestinal morphogenesis and intestinal-specific lipid metabolism in mice. Gastroenterology 122, 469–482 (2002).
Ramalho-Santos, M., Melton, D. A. & McMahon, A. P. Hedgehog signals regulate multiple aspects of gastrointestinal development. Development 127, 2763–2772 (2000).
Roberts, D. J. et al. Sonic hedgehog is an endodermal signal inducing Bmp-4 and Hox genes during induction and regionalization of the chick hindgut. Development 121, 3163–3174 (1995).
Roberts, D. J., Smith, D. M., Goff, D. J. & Tabin, C. J. Epithelial-mesenchymal signaling during the regionalization of the chick gut. Development 125, 2791–2801 (1998).
van den Brink, G. R. et al. Indian Hedgehog is an antagonist of Wnt signaling in colonic epithelial cell differentiation. Nature Genet. 36, 277–282 (2004).
Karlsson, L., Lindahl, P., Heath, J. K. & Betsholtz, C. Abnormal gastrointestinal development in PDGF-A and PDGFR-α deficient mice implicates a novel mesenchymal structure with putative instructive properties in villus morphogenesis. Development 127, 3457–3466 (2000).
Haramis, A. P. et al. De novo crypt formation and juvenile polyposis on BMP inhibition in mouse intestine. Science 303, 1684–1686 (2004). Shows that BMP signalling from the villus mesenchyme is necessary to prevent the villus epithelium from adopting a crypt-like proliferative character.
He, X. C. et al. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt–β-catenin signaling. Nature Genet. 36, 1117–1121 (2004).
Howe, J. R. et al. Mutations in the SMAD4/DPC4 gene in juvenile polyposis. Science 280, 1086–1088 (1998).
Howe, J. R. et al. Germline mutations of the gene encoding bone morphogenetic protein receptor 1A in juvenile polyposis. Nature Genet. 28, 184–187 (2001).
Meinhardt, H. & Gierer, A. Pattern formation by local self-activation and lateral inhibition. BioEssays 22, 753–760 (2000).
Gregorieff, A. et al. Expression pattern of Wnt signaling components in the adult intestine. Gastroenterology 129, 626–638 (2005).
Logan, C. Y. & Nusse, R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 20, 781–810 (2004).
Korinek, V. et al. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nature Genet. 19, 379–383 (1998). The first direct evidence that when the Wnt signalling pathway is defective, the intestinal stem-cell population is not maintained.
Pinto, D., Gregorieff, A., Begthel, H. & Clevers, H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 17, 1709–1713 (2003). By forced expression of DKK1, a secreted Wnt inhibitor, this paper shows that Wnt signalling is needed not only to maintain proliferation of the intestinal epithelium, but also to drive the differentiation of secretory cells.
Kuhnert, F. et al. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc. Natl Acad. Sci. USA 101, 266–271 (2004).
Korinek, V. et al. Constitutive transcriptional activation by a β-catenin–Tcf complex in APC−/− colon carcinoma. Science 275, 1784–1787 (1997).
Andreu, P. et al. Crypt-restricted proliferation and commitment to the Paneth cell lineage following Apc loss in the mouse intestine. Development 132, 1443–1451 (2005).
Sansom, O. J. et al. Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration. Genes Dev. 18, 1385–1390 (2004).
Morin, P. J. et al. Activation of β-catenin–Tcf signaling in colon cancer by mutations in β-catenin or APC. Science 275, 1787–1790 (1997).
Su, L. K. et al. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science 256, 668–670 (1992).
Haramis, A. P. et al. Adenomatous polyposis coli-deficient zebrafish are susceptible to digestive tract neoplasia. EMBO Rep. 27 Jan 2006 (10.1038/sj.embor.7400638).
Bjerknes, M. & Cheng, H. Colossal crypts bordering colon adenomas in ApcMin mice express full-length Apc. Am. J. Pathol. 154, 1831–1834 (1999).
Merritt, A. J., Gould, K. A. & Dove, W. F. Polyclonal structure of intestinal adenomas in ApcMin/+ mice with concomitant loss of Apc+ from all tumor lineages. Proc. Natl Acad. Sci. USA 94, 13927–13931 (1997).
Novelli, M. R. et al. Polyclonal origin of colonic adenomas in an XO/XY patient with FAP. Science 272, 1187–1190 (1996).
Xu, Q., Mellitzer, G., Robinson, V. & Wilkinson, D. G. In vivo cell sorting in complementary segmental domains mediated by Eph receptors and ephrins. Nature 399, 267–271 (1999).
Winslow, J. W. et al. Cloning of AL-1, a ligand for an Eph-related tyrosine kinase receptor involved in axon bundle formation. Neuron 14, 973–981 (1995).
van de Wetering, M. et al. The β-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 111, 241–250 (2002).
Batlle, E. et al. β-Catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell 111, 251–263 (2002). A screen for genes that are misregulated in Wnt pathway mutants identifies genes of the EphB and ephrin B families as targets of Wnt signalling in the intestinal epithelium. Knockouts of these genes show that EphB/ephrin B signalling controls the positioning of cell types along the crypt–villus axis.
Batlle, E. et al. EphB receptor activity suppresses colorectal cancer progression. Nature 435, 1126–1130 (2005).
van Es, J. H. et al. Wnt signalling induces maturation of Paneth cells in intestinal crypts. Nature Cell Biol. 7, 381–386 (2005).
Schroder, N. & Gossler, A. Expression of Notch pathway components in fetal and adult mouse small intestine. Gene Expr. Patterns 2, 247–250 (2002).
Jensen, J. et al. Control of endodermal endocrine development by Hes-1. Nature Genet. 24, 36–44 (2000). One of the first papers to show that Notch signalling affects cell fate choices in the intestinal epithelium.
Crosnier, C. et al. Delta–Notch signalling controls commitment to a secretory fate in the zebrafish intestine. Development 132, 1093–1104 (2005).
van Es, J. H. et al. Notch/γ-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature 435, 959–963 (2005). By conditional knockout of the Notch pathway effector RBPSUH, and also by blocking Notch signalling with a γ-secretase inhibitor, this paper shows that Notch signalling is needed to maintain the proliferative intestinal stem or progenitor population and to prevent all these cells from differentiating as secretory cells.
Milano, J. et al. Modulation of notch processing by γ-secretase inhibitors causes intestinal goblet cell metaplasia and induction of genes known to specify gut secretory lineage differentiation. Toxicol. Sci. 82, 341–358 (2004).
Itoh, M. et al. Mind bomb is a ubiquitin ligase that is essential for efficient activation of Notch signaling by Delta. Dev. Cell 4, 67–82 (2003).
Fre, S. et al. Notch signals control the fate of immature progenitor cells in the intestine. Nature 435, 964–968 (2005). Conditional overexpression of NICD in the intestinal epithelium is used to show that activation of Notch prevents cell differentiation towards the secretory fate and helps to maintain the proliferative state.
Chitnis, A., Henrique, D., Lewis, J., Ish-Horowicz, D. & Kintner, C. Primary neurogenesis in Xenopus embryos regulated by a homologue of the Drosophila neurogenic gene Delta. Nature 375, 761–766 (1995).
Ben-Arie, N. et al. Functional conservation of atonal and Math1 in the CNS and PNS. Development 127, 1039–1048 (2000).
Bermingham, N. A. et al. Math1: an essential gene for the generation of inner ear hair cells. Science 284, 1837–1841 (1999).
Yang, Q., Bermingham, N. A., Finegold, M. J. & Zoghbi, H. Y. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science 294, 2155–2158 (2001). Shows that Math1 is expressed in secretory cells and their precursors in the intestinal epithelium, and that when it is knocked out these cell types are lost.
Kayahara, T. et al. Candidate markers for stem and early progenitor cells, Musashi-1 and Hes1, are expressed in crypt base columnar cells of mouse small intestine. FEBS Lett. 535, 131–135 (2003).
Zecchini, V., Domaschenz, R., Winton, D. & Jones, P. Notch signaling regulates the differentiation of post-mitotic intestinal epithelial cells. Genes Dev. 19, 1686–1691 (2005).
Henrique, D. et al. Maintenance of neuroepithelial progenitor cells by Delta–Notch signalling in the embryonic chick retina. Curr. Biol. 7, 661–670 (1997).
Scheer, N., Groth, A., Hans, S. & Campos-Ortega, J. A. An instructive function for Notch in promoting gliogenesis in the zebrafish retina. Development 128, 1099–1107 (2001).
Yang, X. et al. Notch activation induces apoptosis in neural progenitor cells through a p53-dependent pathway. Dev. Biol. 269, 81–94 (2004).
Handler, M., Yang, X. & Shen, J. Presenilin-1 regulates neuronal differentiation during neurogenesis. Development 127, 2593–2606 (2000).
Dorsky, R. I., Chang, W. S., Rapaport, D. H. & Harris, W. A. Regulation of neuronal diversity in the Xenopus retina by Delta signalling. Nature 385, 67–70 (1997).
Hatakeyama, J. et al. Hes genes regulate size, shape and histogenesis of the nervous system by control of the timing of neural stem cell differentiation. Development 131, 5539–5550 (2004).
Hitoshi, S. et al. Notch pathway molecules are essential for the maintenance, but not the generation, of mammalian neural stem cells. Genes Dev. 16, 846–858 (2002).
Nyfeler, Y. et al. Jagged1 signals in the postnatal subventricular zone are required for neural stem cell self-renewal. EMBO J. 24, 3504–3515 (2005).
Kohyama, J. et al. Visualization of spatiotemporal activation of Notch signaling: live monitoring and significance in neural development. Dev. Biol. 286, 311–325 (2005).
Megason, S. G. & McMahon, A. P. A mitogen gradient of dorsal midline Wnts organizes growth in the CNS. Development 129, 2087–2098 (2002).
Zechner, D. et al. β-Catenin signals regulate cell growth and the balance between progenitor cell expansion and differentiation in the nervous system. Dev. Biol. 258, 406–418 (2003).
Chenn, A. & Walsh, C. A. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science 297, 365–369 (2002).
Lie, D. C. et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature 437, 1370–1375 (2005).
Kubo, F., Takeichi, M. & Nakagawa, S. Wnt2b inhibits differentiation of retinal progenitor cells in the absence of Notch activity by downregulating the expression of proneural genes. Development 132, 2759–2770 (2005).
Cairns, J. Mutation selection and the natural history of cancer. Nature 255, 197–200 (1975).
Potten, C. S., Owen, G. & Booth, D. Intestinal stem cells protect their genome by selective segregation of template DNA strands. J. Cell Sci. 115, 2381–2388 (2002).
Stappenbeck, T. S., Mills, J. C. & Gordon, J. I. Molecular features of adult mouse small intestinal epithelial progenitors. Proc. Natl Acad. Sci. USA 100, 1004–1009 (2003).
Subramanian, V., Meyer, B. & Evans, G. S. The murine Cdx1 gene product localises to the proliferative compartment in the developing and regenerating intestinal epithelium. Differentiation 64, 11–18 (1998).
Potten, C. S. et al. Identification of a putative intestinal stem cell and early lineage marker; musashi-1. Differentiation 71, 28–41 (2003).
Bettess, M. D. et al. c-Myc is required for the formation of intestinal crypts but dispensable for homeostasis of the adult intestinal epithelium. Mol. Cell. Biol. 25, 7868–7878 (2005).
Merok, J. R., Lansita, J. A., Tunstead, J. R. & Sherley, J. L. Cosegregation of chromosomes containing immortal DNA strands in cells that cycle with asymmetric stem cell kinetics. Cancer Res. 62, 6791–6795 (2002).
Karpowicz, P. et al. Support for the immortal strand hypothesis: neural stem cells partition DNA asymmetrically in vitro. J. Cell Biol. 170, 721–732 (2005).
Smith, G. H. Label-retaining epithelial cells in mouse mammary gland divide asymmetrically and retain their template DNA strands. Development 132, 681–687 (2005).
Lowell, S., Jones, P., Le Roux, I., Dunne, J. & Watt, F. M. Stimulation of human epidermal differentiation by delta-notch signalling at the boundaries of stem-cell clusters. Curr. Biol. 10, 491–500 (2000).
Nicolas, M. et al. Notch1 functions as a tumor suppressor in mouse skin. Nature Genet. 33, 416–421 (2003).
Alonso, L. & Fuchs, E. Stem cells in the skin: waste not, Wnt not. Genes Dev. 17, 1189–1200 (2003).
Watt, F. M. Unexpected Hedgehog–Wnt interactions in epithelial differentiation. Trends Mol. Med. 10, 577–580 (2004).
Micchelli, C. A. & Perrimon, N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature 439, 475–479 (2006).
Ohlstein, B. & Spradling, A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature 439, 470–474 (2006).
Duncan, A. W. et al. Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nature Immunol. 6, 314–322 (2005). This paper uses transgenic mice that contain reporter genes to show that in haemopoietic stem cells the Notch and Wnt pathways are both active and serve jointly to maintain the proliferative pluripotent stem-cell state.
Reya, T. et al. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature 423, 409–414 (2003).
Varnum-Finney, B. et al. Pluripotent, cytokine-dependent, hematopoietic stem cells are immortalized by constitutive Notch1 signaling. Nature Med. 6, 1278–1281 (2000).
Karanu, F. N. et al. The notch ligand jagged-1 represents a novel growth factor of human hematopoietic stem cells. J. Exp. Med. 192, 1365–1372 (2000).
Pack, M. et al. Mutations affecting development of zebrafish digestive organs. Development 123, 321–328 (1996).
Grapin-Botton, A. & Melton, D. A. Endoderm development: from patterning to organogenesis. Trends Genet. 16, 124–130 (2000).
Wallace, K. N., Akhter, S., Smith, E. M., Lorent, K. & Pack, M. Intestinal growth and differentiation in zebrafish. Mech. Dev. 122, 157–173 (2005).
Pinto, D., Robine, S., Jaisser, F., El Marjou, F. E. & Louvard, D. Regulatory sequences of the mouse villin (Vill) gene that efficiently drive transgenic expression in immature and differentiated epithelial cells of small and large intestines. J. Biol. Chem. 274, 6476–6482 (1999).
Robine, S., Jaisser, F. & Louvard, D. Epithelial cell growth and differentiation. IV. Controlled spatiotemporal expression of transgenes: new tools to study normal and pathological states. Am. J. Physiol. 273, G759–762 (1997).
el Marjou, F. et al. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis 39, 186–193 (2004).
Ireland, H. et al. Inducible Cre-mediated control of gene expression in the murine gastrointestinal tract: effect of loss of β-catenin. Gastroenterology 126, 1236–1246 (2004).
Acknowledgements
We thank N. Wright and the anonymous referees for helpful comments, and Cancer Research UK for financial support.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Niche
-
The specific microenvironment that stem cells inhabit.
- Clonal analysis
-
Analysis of the composition and distribution of the clones of cells that are descended from individual, heritably marked cells, in order to discover the fate of these progenitors.
- Pseudostratified
-
Describes an epithelium that, because of the uneven positions of the cell nuclei, seems to contain several layers of cells (stratified) but is in fact composed of a single one, in which all cells make contact with the basal surface.
Rights and permissions
About this article
Cite this article
Crosnier, C., Stamataki, D. & Lewis, J. Organizing cell renewal in the intestine: stem cells, signals and combinatorial control. Nat Rev Genet 7, 349–359 (2006). https://doi.org/10.1038/nrg1840
Issue Date:
DOI: https://doi.org/10.1038/nrg1840
This article is cited by
-
Increased Intestinal Permeability: An Avenue for the Development of Autoimmune Disease?
Exposure and Health (2024)
-
Phenotypic heterogeneity in psoriatic arthritis: towards tissue pathology-based therapy
Nature Reviews Rheumatology (2023)
-
Effects of dietary supplementation of bovine lactoferrin on growth performance, immune function and intestinal health in weaning piglets
BioMetals (2023)
-
APOBEC mutagenesis is a common process in normal human small intestine
Nature Genetics (2023)
-
Low-density lipoprotein receptor-related protein 1 is a CROPs-associated receptor for Clostridioides infection toxin B
Science China Life Sciences (2022)