Abstract
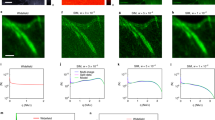
By combining astigmatism imaging with a dual-objective scheme, we improved the image resolution of stochastic optical reconstruction microscopy (STORM) and obtained <10-nm lateral resolution and <20-nm axial resolution when imaging biological specimens. Using this approach, we resolved individual actin filaments in cells and revealed three-dimensional ultrastructure of the actin cytoskeleton. We observed two vertically separated layers of actin networks with distinct structural organizations in sheet-like cell protrusions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hell, S.W. Science 316, 1153–1158 (2007).
Huang, B., Babcock, H. & Zhuang, X.W. Cell 143, 1047–1058 (2010).
Betzig, E. et al. Science 313, 1642–1645 (2006).
Gould, T.J. et al. Nat. Methods 5, 1027–1030 (2008).
Heilemann, M. et al. Angew. Chem. Int. Ed. 47, 6172–6176 (2008).
Vogelsang, J., Cordes, T., Forthmann, C., Steinhauer, C. & Tinnefeld, P. Proc. Natl. Acad. Sci. USA 106, 8107–8112 (2009).
Chhabra, E.S. & Higgs, H.N. Nat. Cell Biol. 9, 1110–1121 (2007).
Pollard, T.D. & Borisy, G.G. Cell 112, 453–465 (2003).
Svitkina, T. Methods Cell Biol. 79, 295–319 (2007).
Urban, E., Jacob, S., Nemethova, M., Resch, G.P. & Small, J.V. Nat. Cell Biol. 12, 429–435 (2010).
Rust, M.J., Bates, M. & Zhuang, X.W. Nat. Methods 3, 793–795 (2006).
Huang, B., Wang, W.Q., Bates, M. & Zhuang, X.W. Science 319, 810–813 (2008).
Shtengel, G. et al. Proc. Natl. Acad. Sci. USA 106, 3125–3130 (2009).
Aquino, D. et al. Nat. Methods 8, 353–359 (2011).
Zhuang, X.W. Nat. Photonics 3, 365–367 (2009).
Pellegrin, S. & Mellor, H. J. Cell Sci. 120, 3491–3499 (2007).
Geiger, B., Spatz, J.P. & Bershadsky, A.D. Nat. Rev. Mol. Cell Biol. 10, 21–33 (2009).
Svitkina, T.M. & Borisy, G.G. J. Cell Biol. 145, 1009–1026 (1999).
Giannone, G. et al. Cell 128, 561–575 (2007).
Small, J.V., Rottner, K., Hahne, P. & Anderson, K.I. Microsc. Res. Tech. 47, 3–17 (1999).
Koestler, S.A., Auinger, S., Vinzenz, M., Rottner, K. & Small, J.V. Nat. Cell Biol. 10, 306–313 (2008).
Auinger, S. & Small, J.V. Methods Cell Biol. 88, 257–272 (2008).
Dempsey, G.T. et al. J. Am. Chem. Soc. 131, 18192–18193 (2009).
Goshtasby, A. 2-D and 3-D Image Registration for Medical, Remote Sensing, and Industrial Applications (Wiley, 2005).
Acknowledgements
We thank G. Danuser for helpful discussion. This work was supported in part by the US National Institutes of Health and a Collaborative Innovation Award (43667) from Howard Hughes Medical Institute and Gatsby Charitable Foundation (to X.Z.). X.Z. is funded by the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
K.X., H.P.B. and X.Z. designed research. K.X. did experiments and data analysis. H.P.B. assisted with the optical setup. K.X. and X.Z. prepared the manuscript. X.Z. supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7, Supplementary Results, Supplementary Discussion and Supplementary Protocols 1–2 (PDF 23681 kb)
Supplementary Software
Analysis software (ZIP 4 kb)
Rights and permissions
About this article
Cite this article
Xu, K., Babcock, H. & Zhuang, X. Dual-objective STORM reveals three-dimensional filament organization in the actin cytoskeleton. Nat Methods 9, 185–188 (2012). https://doi.org/10.1038/nmeth.1841
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.1841
This article is cited by
-
HOPE-SIM, a cryo-structured illumination fluorescence microscopy system for accurately targeted cryo-electron tomography
Communications Biology (2023)
-
Maximum-likelihood model fitting for quantitative analysis of SMLM data
Nature Methods (2023)
-
Quantitatively mapping local quality of super-resolution microscopy by rolling Fourier ring correlation
Light: Science & Applications (2023)
-
4polar-STORM polarized super-resolution imaging of actin filament organization in cells
Nature Communications (2022)
-
Simple methods for quantifying super-resolved cortical actin
Scientific Reports (2022)