Abstract
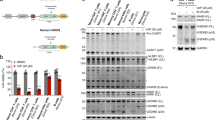
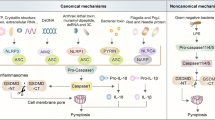
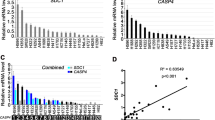
Val-boroPro (Talabostat, PT-100), a nonselective inhibitor of post-proline cleaving serine proteases, stimulates mammalian immune systems through an unknown mechanism of action. Despite this lack of mechanistic understanding, Val-boroPro has attracted substantial interest as a potential anticancer agent, reaching phase 3 trials in humans. Here we show that Val-boroPro stimulates the immune system by triggering a proinflammatory form of cell death in monocytes and macrophages known as pyroptosis. We demonstrate that the inhibition of two serine proteases, DPP8 and DPP9, activates the pro-protein form of caspase-1 independent of the inflammasome adaptor ASC. Activated pro-caspase-1 does not efficiently process itself or IL-1β but does cleave and activate gasdermin D to induce pyroptosis. Mice lacking caspase-1 do not show immune stimulation after treatment with Val-boroPro. Our data identify what is to our knowledge the first small molecule that induces pyroptosis and reveals a new checkpoint that controls the activation of the innate immune system.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Zitvogel, L., Tesniere, A. & Kroemer, G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat. Rev. Immunol. 6, 715–727 (2006).
Dunn, G.P., Bruce, A.T., Ikeda, H., Old, L.J. & Schreiber, R.D. Cancer immunoediting: from immunosurveillance to tumor escape. Nat. Immunol. 3, 991–998 (2002).
Topalian, S.L. et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366, 2443–2454 (2012).
Wolchok, J.D. et al. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 369, 122–133 (2013).
Sharma, P. & Allison, J.P. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell 161, 205–214 (2015).
Couzin-Frankel, J. Breakthrough of the year 2013. Cancer immunotherapy. Science 342, 1432–1433 (2013).
Adams, S. et al. PT-100, a small molecule dipeptidyl peptidase inhibitor, has potent antitumor effects and augments antibody-mediated cytotoxicity via a novel immune mechanism. Cancer Res. 64, 5471–5480 (2004).
Walsh, M.P. et al. Val-boroPro accelerates T cell priming via modulation of dendritic cell trafficking resulting in complete regression of established murine tumors. PLoS One 8, e58860 (2013).
Jones, B. et al. Hematopoietic stimulation by a dipeptidyl peptidase inhibitor reveals a novel regulatory mechanism and therapeutic treatment for blood cell deficiencies. Blood 102, 1641–1648 (2003).
Jesson, M.I. et al. Immune mechanism of action of talabostat: a dipeptidyl peptidase targeted antitumor agent. in Proceedings of the 98th Annual Meeting of the American Association for Cancer Research abstr. 1984 (2007).
Kayagaki, N. et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526, 666–671 (2015).
Broz, P. Immunology: Caspase target drives pyroptosis. Nature 526, 642–643 (2015).
Kortmann, J., Brubaker, S.W. & Monack, D.M. Cutting Edge: Inflammasome Activation in Primary Human Macrophages Is Dependent on Flagellin. J. Immunol. 195, 815–819 (2015).
Lankas, G.R. et al. Dipeptidyl peptidase IV inhibition for the treatment of type 2 diabetes: potential importance of selectivity over dipeptidyl peptidases 8 and 9. Diabetes 54, 2988–2994 (2005).
Bachovchin, D.A. et al. A high-throughput, multiplexed assay for superfamily-wide profiling of enzyme activity. Nat. Chem. Biol. 10, 656–663 (2014).
Ohnuma, K. et al. CD26 up-regulates expression of CD86 on antigen-presenting cells by means of caveolin-1. Proc. Natl. Acad. Sci. USA 101, 14186–14191 (2004).
Lee, H.J. et al. Investigation of the dimer interface and substrate specificity of prolyl dipeptidase DPP8. J. Biol. Chem. 281, 38653–38662 (2006).
Tang, H.K. et al. Biochemical properties and expression profile of human prolyl dipeptidase DPP9. Arch. Biochem. Biophys. 485, 120–127 (2009).
Ajami, K., Abbott, C.A., McCaughan, G.W. & Gorrell, M.D. Dipeptidyl peptidase 9 has two forms, a broad tissue distribution, cytoplasmic localization and DPIV-like peptidase activity. Biochim. Biophys. Acta 1679, 18–28 (2004).
Abbott, C.A. et al. Cloning, expression and chromosomal localization of a novel human dipeptidyl peptidase (DPP) IV homolog, DPP8. Eur. J. Biochem. 267, 6140–6150 (2000).
Zhang, H., Chen, Y., Keane, F.M. & Gorrell, M.D. Advances in understanding the expression and function of dipeptidyl peptidase 8 and 9. Mol. Cancer Res. 11, 1487–1496 (2013).
Waumans, Y., Baerts, L., Kehoe, K., Lambeir, A.M. & De Meester, I. The dipeptidyl peptidase family, prolyl oligopeptidase, and prolyl carboxypeptidase in the immune system and inflammatory disease, including atherosclerosis. Front. Immunol. 6, 387 (2015).
Wagner, L., Klemann, C., Stephan, M. & von Hörsten, S. Unravelling the immunological roles of dipeptidyl peptidase 4 (DPP4) activity and/or structure homologue (DASH) proteins. Clin. Exp. Immunol. 184, 265–283 (2016).
Jiaang, W.T. et al. Novel isoindoline compounds for potent and selective inhibition of prolyl dipeptidase DPP8. Bioorg. Med. Chem. Lett. 15, 687–691 (2005).
Wu, J.J. et al. Biochemistry, pharmacokinetics, and toxicology of a potent and selective DPP8/9 inhibitor. Biochem. Pharmacol. 78, 203–210 (2009).
Waumans, Y. et al. The dipeptidyl peptidases 4, 8, and 9 in mouse monocytes and macrophages: DPP8/9 inhibition attenuates M1 macrophage activation in mice. Inflammation 39, 413–424 (2016).
Lamkanfi, M. & Dixit, V.M. Mechanisms and functions of inflammasomes. Cell 157, 1013–1022 (2014).
Shi, J. et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514, 187–192 (2014).
Augeri, D.J. et al. Discovery and preclinical profile of Saxagliptin (BMS-477118): a highly potent, long-acting, orally active dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. J. Med. Chem. 48, 5025–5037 (2005).
Villhauer, E.B. et al. 1-[[(3-hydroxy-1-adamantyl)amino]acetyl]-2-cyano-(S)-pyrrolidine: a potent, selective, and orally bioavailable dipeptidyl peptidase IV inhibitor with antihyperglycemic properties. J. Med. Chem. 46, 2774–2789 (2003).
Matheeussen, V. et al. Dipeptidyl peptidases in atherosclerosis: expression and role in macrophage differentiation, activation and apoptosis. Basic Res. Cardiol. 108, 350 (2013).
Shi, J. et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 (2015).
Pelegrin, P., Barroso-Gutierrez, C. & Surprenant, A. P2X7 receptor differentially couples to distinct release pathways for IL-1β in mouse macrophage. J. Immunol. 180, 7147–7157 (2008).
Broz, P., von Moltke, J., Jones, J.W., Vance, R.E. & Monack, D.M. Differential requirement for caspase-1 autoproteolysis in pathogen-induced cell death and cytokine processing. Cell Host Microbe 8, 471–483 (2010).
Van Opdenbosch, N. et al. Activation of the NLRP1b inflammasome independently of ASC-mediated caspase-1 autoproteolysis and speck formation. Nat. Commun. 5, 3209 (2014).
Guey, B., Bodnar, M., Manié, S.N., Tardivel, A. & Petrilli, V. Caspase-1 autoproteolysis is differentially required for NLRP1b and NLRP3 inflammasome function. Proc. Natl. Acad. Sci. USA 111, 17254–17259 (2014).
He, W.T. et al. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 25, 1285–1298 (2015).
Kayagaki, N. et al. Non-canonical inflammasome activation targets caspase-11. Nature 479, 117–121 (2011).
Wilson, C.H. et al. Identifying natural substrates for dipeptidyl peptidases 8 and 9 using terminal amine isotopic labeling of substrates (TAILS) reveals in vivo roles in cellular homeostasis and energy metabolism. J. Biol. Chem. 288, 13936–13949 (2013).
Zhang, H. et al. Identification of novel dipeptidyl peptidase 9 substrates by two-dimensional differential in-gel electrophoresis. FEBS J. 282, 3737–3757 (2015).
Burkey, B.F. et al. Adverse effects of dipeptidyl peptidases 8 and 9 inhibition in rodents revisited. Diabetes Obes. Metab. 10, 1057–1061 (2008).
Rosenblum, J.S., Liu, Y., Wu, J. & Kozarich, J.W. The case against toxicity from DPP8/9 inhibition. in American Diabetes Association Conference (2007).
Doench, J.G. et al. Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR-Cas9. Nat. Biotechnol. 34, 184–191 (2016).
Sanjana, N.E., Shalem, O. & Zhang, F. Improved vectors and genome-wide libraries for CRISPR screening. Nat. Methods 11, 783–784 (2014).
Coutts, S.J. et al. Structure-activity relationships of boronic acid inhibitors of dipeptidyl peptidase IV. 1. Variation of the P2 position of Xaa-boroPro dipeptides. J. Med. Chem. 39, 2087–2094 (1996).
Danilova, O., Li, B., Szardenings, A.K., Huber, B.T. & Rosenblum, J.S. Synthesis and activity of a potent, specific azabicyclo[3.3.0]-octane-based DPP II inhibitor. Bioorg. Med. Chem. Lett. 17, 507–510 (2007).
Wang, X.M., Yu, D.M., McCaughan, G.W. & Gorrell, M.D. Fibroblast activation protein increases apoptosis, cell adhesion, and migration by the LX-2 human stellate cell line. Hepatology 42, 935–945 (2005).
Poplawski, S.E. et al. Identification of selective and potent inhibitors of fibroblast activation protein and prolyl oligopeptidase. J. Med. Chem. 56, 3467–3477 (2013).
Acknowledgements
We thank S. Fujisawa for microscopy assistance, E. De Stanchina and B. Qeriqi for assistance harvesting mBMDMs, C. Taabazuing for helpful comments and A. Kentsis and F. Brown (Molecular Pharmacology Program, Memorial Sloan Kettering Cancer Center) for the HL-60 cell line. This work was supported by the Josie Robertson Foundation (D.A.B.), the MSKCC Core Grant (P30 CA008748), the NCI (grant no. U54CA112962 to T.R.G.), HHMI (T.R.G.) and the NIH (CA174008-01A1 to W.W.B. and NIH NIGMS T32 GM115327-Tan to D.C.J.).
Author information
Authors and Affiliations
Contributions
D.A.B. conceived and directed the project, performed experiments, analyzed data and wrote the paper; M.C.O., D.C.J., R.S., E.B.G., A.J.C. and M.S.W. performed experiments and analyzed data; S.E.P. performed the in vivo mouse experiments and the intracellular DPP8/9 inhibition experiment; W.W.B. and D.G.S. directed the in vivo mouse experiments; W.W., Y.L. and J.H.L. synthesized Val-boroPro, 1G244, L-allo-isoleucin-isoindoline, L-allo-isoleucine-thiazolidine, compound 5385 and FP-biotin; M.O.A. characterized compound 5385. T.R.G. and W.W.B. helped plan the study.
Corresponding author
Ethics declarations
Competing interests
W.W.B. is a co-founder, advisor and board member of Arisaph Pharmaceuticals, a biotechnology company interested in developing boronic acid–based inhibitors of serine proteases as therapeutics.
Supplementary information
Supplementary Text and Figures
Supplementary Results, Supplementary Tables 1 and 2 and Supplementary Figures 1–12. (PDF 14969 kb)
Supplementary Video 1
DMSO-treated RAW 264.7 cells. (MOV 17579 kb)
Supplementary Video 2
Etoposide-induced apoptosis in RAW 264.7 cells. (MOV 13930 kb)
Supplementary Video 3
Val-boroPro-induced pyroptosis in RAW 264.7 cells. (MOV 15726 kb)
Supplementary Video 4
1G244-induced pyroptosis in RAW 264.7 cells. (MOV 13860 kb)
Rights and permissions
About this article
Cite this article
Okondo, M., Johnson, D., Sridharan, R. et al. DPP8 and DPP9 inhibition induces pro-caspase-1-dependent monocyte and macrophage pyroptosis. Nat Chem Biol 13, 46–53 (2017). https://doi.org/10.1038/nchembio.2229
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.2229
This article is cited by
-
A palmitoylation–depalmitoylation relay spatiotemporally controls GSDMD activation in pyroptosis
Nature Cell Biology (2024)
-
New prospects of cancer therapy based on pyroptosis and pyroptosis inducers
Apoptosis (2024)
-
Involvement of inflammasomes in tumor microenvironment and tumor therapies
Journal of Hematology & Oncology (2023)
-
Investigation into the role of the MITA-TRIM38 interaction in regulating pyroptosis and maintaining immune tolerance at the maternal-fetal interface
Cell Death & Disease (2023)
-
NLRP1- A CINDERELLA STORY: a perspective of recent advances in NLRP1 and the questions they raise
Communications Biology (2023)