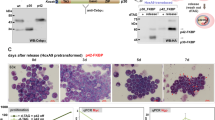
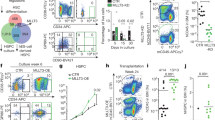
Abstract
In blood, the transcription factor C/EBPa is essential for myeloid differentiation and has been implicated in regulating self-renewal of fetal liver haematopoietic stem cells (HSCs). However, its function in adult HSCs has remained unknown. Here, using an inducible knockout model we found that C/EBPa-deficient adult HSCs underwent a pronounced increase in number with enhanced proliferation, characteristics resembling fetal liver HSCs. Consistently, transcription profiling of C/EBPa-deficient HSCs revealed a gene expression program similar to fetal liver HSCs. Moreover, we observed that age-specific Cebpa expression correlated with its inhibitory effect on the HSC cell cycle. Mechanistically we identified N-Myc as a downstream target of C/EBPa, and loss of C/EBPa resulted in de-repression of N-Myc. Our data establish C/EBPa as a central determinant in the switch from fetal to adult HSCs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Accession codes
References
Morrison, S. J., Hemmati, H. D., Wandycz, A. M. & Weissman, I. L. The purification and characterization of fetal liver hematopoietic stem cells. Proc. Natl Acad. Sci. USA 92, 10302–10306 (1995).
Ema, H. & Nakauchi, H. Expansion of hematopoietic stem cells in the developing liver of a mouse embryo. Blood 95, 2284–2288 (2000).
Morrison, S. J. & Weissman, I. L. The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity 1, 661–673 (1994).
Goodell, M. A., Brose, K., Paradis, G., Conner, A. S. & Mulligan, R. C. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J. Exp. Med. 183, 1797–1806 (1996).
Cheshier, S. H., Morrison, S. J., Liao, X. & Weissman, I. L. In vivo proliferation and cell cycle kinetics of long-term self-renewing hematopoietic stem cells. Proc. Natl Acad. Sci. USA 96, 3120–3125 (1999).
Passegue, E., Wagers, A. J., Giuriato, S., Anderson, W. C. & Weissman, I. L. Global analysis of proliferation and cell cycle gene expression in the regulation of hematopoietic stem and progenitor cell fates. J. Exp. Med. 202, 1599–1611 (2005).
Wilson, A. et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell 135, 1118–1129 (2008).
Bowie, M. B. et al. Hematopoietic stem cells proliferate until after birth and show a reversible phase-specific engraftment defect. J. Clin. Invest. 116, 2808–2816 (2006).
Harrison, D. E., Zhong, R. K., Jordan, C. T., Lemischka, I. R. & Astle, C. M. Relative to adult marrow, fetal liver repopulates nearly five times more effectively long-term than short-term. Exp. Hematol. 25, 293–297 (1997).
Bowie, M. B. et al. Identification of a new intrinsically timed developmental checkpoint that reprograms key hematopoietic stem cell properties. Proc. Natl Acad. Sci. USA 104, 5878–5882 (2007).
Kim, I., Saunders, T. L. & Morrison, S. J. Sox17 dependence distinguishes the transcriptional regulation of fetal from adult hematopoietic stem cells. Cell 130, 470–483 (2007).
Park, I. K. et al. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature 423, 302–305 (2003).
Hock, H. et al. Gfi-1 restricts proliferation and preserves functional integrity of haematopoietic stem cells. Nature 431, 1002–1007 (2004).
Hock, H. et al. Tel/Etv6 is an essential and selective regulator of adult hematopoietic stem cell survival. Genes Dev. 18, 2336–2341 (2004).
Johnson, P. F. Molecular stop signs: regulation of cell-cycle arrest by C/EBP transcription factors. J. Cell Sci. 118, 2545–2555 (2005).
Nerlov, C. The C/EBP family of transcription factors: a paradigm for interaction between gene expression and proliferation control. Trends Cell Biol. 17, 318–324 (2007).
McKnight, S. L. McBindall–a better name for CCAAT/enhancer binding proteins? Cell 107, 259–261 (2001).
Zhang, D. E. et al. Absence of granulocyte colony-stimulating factor signaling and neutrophil development in CCAAT enhancer binding protein α-deficient mice. Proc. Natl Acad. Sci. USA 94, 569–574 (1997).
Zhang, P. et al. Enhancement of hematopoietic stem cell repopulating capacity and self-renewal in the absence of the transcription factor C/EBP α. Immunity 21, 853–863 (2004).
Pabst, T. et al. Dominant-negative mutations of CEBPA, encoding CCAAT/enhancer binding protein- α (C/EBPα), in acute myeloid leukemia. Nat. Genet. 27, 263–270 (2001).
Wouters, B. J. et al. Distinct gene expression profiles of acute myeloid/T-lymphoid leukemia with silenced CEBPA and mutations in NOTCH1. Blood 110, 3706–3714 (2007).
Kiel, M. J., Yilmaz, O. H., Iwashita, T., Terhorst, C. & Morrison, S. J. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121, 1109–1121 (2005).
Kim, I., He, S., Yilmaz, O. H., Kiel, M. J. & Morrison, S. J. Enhanced purification of fetal liver hematopoietic stem cells using SLAM family receptors. Blood 108, 737–744 (2006).
Szilvassy, S. J., Humphries, R. K., Lansdorp, P. M., Eaves, A. C. & Eaves, C. J. Quantitative assay for totipotent reconstituting hematopoietic stem cells by a competitive repopulation strategy. Proc. Natl Acad. Sci. USA 87, 8736–8740 (1990).
Essers, M. A. et al. IFNα activates dormant haematopoietic stem cells in vivo. Nature 458, 904–908 (2009).
Venezia, T. A. et al. Molecular signatures of proliferation and quiescence in hematopoietic stem cells. PLoS Biol. 2, e301 (2004).
He, S., Kim, I., Lim, M. S. & Morrison, S. J. Sox17 expression confers self-renewal potential and fetal stem cell characteristics upon adult hematopoietic progenitors. Genes Dev. 25, 1613–1627 (2011).
Laurenti, E. et al. Hematopoietic stem cell function and survival depend on c-Myc and N-Myc activity. Cell Stem Cell 3, 611–624 (2008).
Bowie, M. B., Kent, D. G., Copley, M. R. & Eaves, C. J. Steel factor responsiveness regulates the high self-renewal phenotype of fetal hematopoietic stem cells. Blood 109, 5043–5048 (2007).
Iwama, A. et al. Enhanced self-renewal of hematopoietic stem cells mediated by the polycomb gene product Bmi-1. Immunity 21, 843–851 (2004).
Ikuta, K. et al. A developmental switch in thymic lymphocyte maturation potential occurs at the level of hematopoietic stem cells. Cell 62, 863–874 (1990).
Kikuchi, K. & Kondo, M. Developmental switch of mouse hematopoietic stem cells from fetal to adult type occurs in bone marrow after birth. Proc. Natl Acad. Sci. USA 103, 17852–17857 (2006).
Meyer, N. & Penn, L. Z. Reflecting on 25 years with MYC. Nat. Rev. Cancer 8, 976–990 (2008).
Freytag, S. O. & Geddes, T. J. Reciprocal regulation of adipogenesis by Myc and C/EBP α. Science 256, 379–382 (1992).
Johansen, L. M. et al. c-Myc is a critical target for c/EBPα in granulopoiesis. Mol. Cell Biol. 21, 3789–3806 (2001).
Sawai, S. et al. Defects of embryonic organogenesis resulting from targeted disruption of the N-myc gene in the mouse. Development 117, 1445–1455 (1993).
Stanton, B. R., Perkins, A. S., Tessarollo, L., Sassoon, D. A. & Parada, L. F. Loss of N-myc function results in embryonic lethality and failure of the epithelial component of the embryo to develop. Genes Dev. 6, 2235–2247 (1992).
Hirai, H. et al. C/EBPβ is required for ‘emergency’ granulopoiesis. Nat. Immunol. 7, 732–229 (2006).
Santaguida, M. et al. JunB protects against myeloid malignancies by limiting hematopoietic stem cell proliferation and differentiation without affecting self-renewal. Cancer Cell 15, 341–352 (2009).
Irizarry, R. A., Ooi, S. L., Wu, Z. & Boeke, J. D. Use of mixture models in a microarray-based screening procedure for detecting differentially represented yeast mutants. Stat. Appl. Genet Mol. Biol. 2, 1 (2003).
Chua, S. W. et al. A novel normalization method for effective removal of systematic variation in microarray data. Nucl. Acids Res. 34, e38 (2006).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Acknowledgements
This study was supported by the National Institutes of Health grant HL 56745 and the Harvard Stem Cell Institute grant DP-0086-10-00. G.A. was supported by the Collegio Ghislieri Fellowship Program. E.L. was supported by FAMRI YCSA and CIA grants. P.B.S. was supported by the Austrian Research Foundation and the European Union. D.G.T. is supported by the Singapore Ministry of Health’s National Medical Research Council under its Singapore Translational Research (STaR) Investigator Award. We thank all members of the Tenen laboratory for helpful discussions; R. Welner, C. Bach, H. Xie, M. Stadtfeld, T. Graf and X. Guo for careful reading of the manuscript and suggestions; J. LaVecchio and G. Buruzula from the Harvard Stem Cell Institute/Joslin Diabetes Center flow cytometry facility for their expertise during cell sorting; and A. T. Lay Keng and L. M. Hui from the NUS-Duke genomic facility in Singapore for their expertise in microarray analysis.
Author information
Authors and Affiliations
Contributions
M.Y. and D.G.T. designed the study; M.Y., H.Z., G.A., H.Y., P.Z., E.L., P.B.S., J.Z. and M.A.J. performed research; M.Y., H.Z., H.Y. and P.Z. analysed data; M.Y., H.Z. and D.G.T. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1194 kb)
Supplementary Table 1
Supplementary Information (XLS 76 kb)
Supplementary Table 2
Supplementary Information (XLS 68 kb)
Supplementary Table 3
Supplementary Information (XLS 68 kb)
Supplementary Table 4
Supplementary Information (XLS 391 kb)
Supplementary Table 5
Supplementary Information (XLS 30 kb)
Supplementary Table 6
Supplementary Information (XLS 18 kb)
Supplementary Table 7
Supplementary Information (XLS 56 kb)
Rights and permissions
About this article
Cite this article
Ye, M., Zhang, H., Amabile, G. et al. C/EBPa controls acquisition and maintenance of adult haematopoietic stem cell quiescence. Nat Cell Biol 15, 385–394 (2013). https://doi.org/10.1038/ncb2698
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2698
This article is cited by
-
Identification and interrogation of the gene regulatory network of CEBPA-double mutant acute myeloid leukemia
Leukemia (2023)
-
Childhood hematopoietic stem cells constitute the permissive window for RUNX1-ETO leukemogenesis
International Journal of Hematology (2023)
-
Cell-intrinsic factors governing quiescence vis-à-vis activation of adult hematopoietic stem cells
Molecular and Cellular Biochemistry (2023)
-
Fbxw11 impairs the repopulation capacity of hematopoietic stem/progenitor cells
Stem Cell Research & Therapy (2022)
-
The Fetal-to-Adult Hematopoietic Stem Cell Transition and its Role in Childhood Hematopoietic Malignancies
Stem Cell Reviews and Reports (2021)