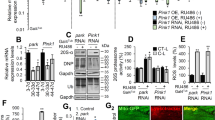
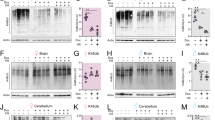
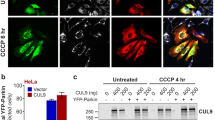
Abstract
Mitochondrial function degenerates with ageing and in ageing-related neuromuscular degenerative diseases, causing physiological decline of the cell1. Factors that can delay the degenerative process are actively sought after. Here, we show that reduced cytosolic protein synthesis is a robust cellular strategy that suppresses ageing-related mitochondrial degeneration. We modelled autosomal dominant progressive external ophthalmoplegia (adPEO), an adult- or later-onset degenerative disease, by introducing the A128P mutation into the adenine nucleotide translocase Aac2p of Saccharomyces cerevisiae. The aac2A128P allele dominantly induces ageing-dependent mitochondrial degeneration and phenotypically tractable degenerative cell death, independently of its ADP/ATP exchange activity. Mitochondrial degeneration was suppressed by lifespan-extending nutritional interventions and by eight longevity mutations, which are all known to reduce cytosolic protein synthesis. These longevity interventions also independently suppressed ageing-related mitochondrial degeneration in the pro-ageing prohibitin mutants. The aac2A128P mutant has reduced mitochondrial membrane potential (Δψm) and is synthetically lethal to low Δψm conditions, including the loss of prohibitin. Mitochondrial degeneration was accelerated by defects in protein turnover on the inner membrane and was suppressed by cycloheximide, a specific inhibitor of cytosolic ribosomes. Reduced cytosolic protein synthesis suppressed membrane depolarization and defects in mitochondrial gene expression in aac2A128P cells. Our finding thus establishes a link between protein homeostasis (proteostasis), cellular bioenergetics and mitochondrial maintenance during ageing.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Wallace, D. C. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu. Rev. Genet. 39, 359–407 (2005).
Spinazzola, A. & Zeviani, M. Disorders of nuclear-mitochondrial intergenomic signaling. Gene 354, 162–168 (2005).
Suomalainen, A. & Kaukonen, J. Diseases caused by nuclear genes affecting mtDNA stability. Am. J. Med. Genet. 106, 53–61 (2001).
Kaukonen, J. et al. Role of adenine nucleotide translocator 1 in mtDNA maintenance. Science 289, 782–785 (2000).
Esposito, L. A., Melov, S., Panov, A., Cottrell, B. A. & Wallace, D. C. Mitochondrial disease in mouse results in increased oxidative stress. Proc. Natl Acad. Sci. USA 96, 4820–4825 (1999).
Fontanesi, F. et al. Mutations in AAC2, equivalent to human adPEO-associated ANT1 mutations, lead to defective oxidative phosphorylation in Saccharomyces cerevisiae and affect mitochondrial DNA stability. Hum. Mol. Genet. 13, 923–934 (2004).
Chen, X. J. Induction of an unregulated channel by mutations in adenine nucleotide translocase suggests an explanation for human ophthalmoplegia. Hum. Mol. Genet. 11, 1835–1843 (2002).
Pebay-Peyroula, E. et al. Structure of mitochondrial ADP/ATP carrier in complex with carboxyatractyloside. Nature 426, 39–44 (2003).
Heidkamper, D., Muller, V., Nelson, D. R. & Klingenberg, M. Probing the role of positive residues in the ADP/ATP carrier from yeast. The effect of six arginine mutations on transport and the four ATP versus ADP exchange modes. Biochemistry 35, 16144–16152 (1996).
Fabrizio, P., Pletcher, S. D., Minois, N., Vaupel, J. W. & Longo, V. D. Chronological aging-independent replicative life span regulation by Msn2/Msn4 and Sod2 in Saccharomyces cerevisiae. FEBS Lett. 557, 136–142 (2004).
Kaeberlein, M. et al. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science 310, 1193–1196 (2005).
Steffen, K. K. et al. Yeast life span extension by depletion of 60S ribosomal subunit is mediated by Gcn4. Cell 133, 292–302 (2008).
Lin, S. J., Defossez, P. A. & Guarente, L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science 289, 2126–2128 (2000).
Sinclair, D. A. & Guarente, L. Extrachromosomal rDNA circles — a cause of aging in yeast. Cell 91, 1033–1042 (1997).
Defossez, P. A. et al. Elimination of replication block protein Fob1 extends the life span of yeast mother cells. Mol. Cell 3, 447–455 (1999).
Lebreton, A. et al. A functional network involved in the recycling of nucleocytoplasmic pre-60S factors. J. Cell Biol. 173, 349–360 (2006).
Jiang, J. C., Jaruga, E., Repnevskaya, M. V. & Jazwinski, S. M. An intervention resembling caloric restriction prolongs life span and retards aging in yeast. FASEB J. 14, 2135–2137 (2000).
Rinnerthaler, M. et al. MMI1 (YKL056c, TMA19), the yeast orthologue of the translationally controlled tumor protein (TCTP) has apoptotic functions and interacts with both microtubules and mitochondria. Biochim. Biophys. Acta 1757, 631–638 (2006).
Kim, S., Benguria, A., Lai, C. Y. & Jazwinski, S. M. Modulation of life-span by histone deacetylase genes in Saccharomyces cerevisiae. Mol Biol Cell 10, 3125–3136 (1999).
Fleischer, T. C., Weaver, C. M., McAfee, K. J., Jennings, J. L. & Link, A. J. Systematic identification and functional screens of uncharacterized proteins associated with eukaryotic ribosomal complexes. Genes Dev. 20, 1294–1307 (2006).
Meskauskas, A. et al. Delayed rRNA processing results in significant ribosome biogenesis and functional defects. Mol. Cell Biol. 23, 1602–1613 (2003).
Lai, C. Y., Jaruga, E., Borghouts, C. & Jazwinski, S. M. A mutation in the ATP2 gene abrogates the age asymmetry between mother and daughter cells of the yeast Saccharomyces cerevisiae. Genetics 162, 73–87 (2002).
Coates, P. J., Jamieson, D. J., Smart, K., Prescott, A. R. & Hall, P. A. The prohibitin family of mitochondrial proteins regulate replicative lifespan. Curr. Biol. 7, 607–610 (1997).
Arnold, I. & Langer, T. Membrane protein degradation by AAA proteases in mitochondria. Biochim. Biophys. Acta 1592, 89–96 (2002).
Chen, J. C., Jiang, C. Z. & Reid, M. S. Silencing a prohibitin alters plant development and senescence. Plant J. 44, 16–24 (2005).
Dunn, C. D., Lee, M. S., Spencer, F. A. & Jensen, R. E. A genomewide screen for petite-negative yeast strains yields a new subunit of the i-AAA protease complex. Mol. Biol. Cell 17, 213–226 (2006).
Thorsness, P. E., White, K. H. & Fox, T. D. Inactivation of YME1, a member of the ftsH-SEC18-PAS1-CDC48 family of putative ATPase-encoding genes, causes increased escape of DNA from mitochondria in Saccharomyces cerevisiae. Mol. Cell Biol. 13, 5418–5426 (1993).
Pearce, D. A. & Sherman, F. Degradation of cytochrome oxidase subunits in mutants of yeast lacking cytochrome c and suppression of the degradation by mutation of yme1. J. Biol. Chem. 270, 20879–20882 (1995).
Brand, M. D. et al. The basal proton conductance of mitochondria depends on adenine nucleotide translocase content. Biochem. J. 392, 353–362 (2005).
Miller, R. A. et al. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell 4, 119–125 (2005).
Lin, S. J. et al. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature 418, 344–348 (2002).
Tong, J. J., Schriner, S. E., McCleary, D., Day, B. J. & Wallace, D. C. Life extension through neurofibromin mitochondrial regulation and antioxidant therapy for neurofibromatosis-1 in Drosophila melanogaster. Nature Genet. 39, 476–485 (2007).
Bonawitz, N. D., Chatenay-Lapointe, M., Pan, Y. & Shadel, G. S. Reduced TOR signaling extends chronological life span via increased respiration and upregulation of mitochondrial gene expression. Cell Metab. 5, 265–277 (2007).
Tavernarakis, N. Ageing and the regulation of protein synthesis: a balancing act? Trends Cell Biol. 18, 228–235 (2008).
Chiocchetti, A. et al. Ribosomal proteins Rpl10 and Rps6 are potent regulators of yeast replicative life span. Exp. Gerontol. 42, 275–286 (2007).
Acknowledgements
We thank M. Schmitt and M. Kucej for critical reading of the manuscript, and L. Sabova and C. Koehler for providing the anti-Aac2 antibody. This work was supported by grants from National Institutes of Health (AG023731) and the American Heart Association (0435047N).
Author information
Authors and Affiliations
Contributions
X.W., X.Z., B.K. and X.J.C. performed the experiments; X.J.C. designed the experiments, analysed data and wrote the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures S1, S2, S3, S4, S5, Supplementary Methods, Supplementary Table S1, Supplementary Note and Supplementary Discussion (PDF 1374 kb)
Rights and permissions
About this article
Cite this article
Wang, X., Zuo, X., Kucejova, B. et al. Reduced cytosolic protein synthesis suppresses mitochondrial degeneration. Nat Cell Biol 10, 1090–1097 (2008). https://doi.org/10.1038/ncb1769
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1769
This article is cited by
-
Sensing, signaling and surviving mitochondrial stress
Cellular and Molecular Life Sciences (2021)
-
Combined flow cytometry and high-throughput image analysis for the study of essential genes in Caenorhabditis elegans
BMC Biology (2018)
-
A cytosolic network suppressing mitochondria-mediated proteostatic stress and cell death
Nature (2015)
-
The mitochondrial unfolded protein response in mammalian physiology
Mammalian Genome (2014)
-
Chemical-genetic profile analysis of five inhibitory compounds in yeast
BMC Chemical Biology (2010)