Abstract
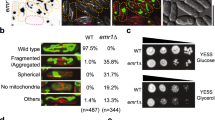
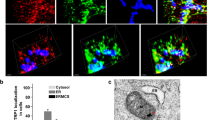
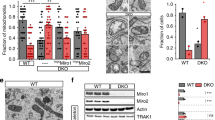
Juxtaposition between endoplasmic reticulum (ER) and mitochondria is a common structural feature, providing the physical basis for intercommunication during Ca2+ signalling; yet, the molecular mechanisms controlling this interaction are unknown. Here we show that mitofusin 2, a mitochondrial dynamin-related protein mutated in the inherited motor neuropathy Charcot–Marie–Tooth type IIa, is enriched at the ER–mitochondria interface. Ablation or silencing of mitofusin 2 in mouse embryonic fibroblasts and HeLa cells disrupts ER morphology and loosens ER–mitochondria interactions, thereby reducing the efficiency of mitochondrial Ca2+ uptake in response to stimuli that generate inositol-1,4,5-trisphosphate. An in vitro assay as well as genetic and biochemical evidences support a model in which mitofusin 2 on the ER bridges the two organelles by engaging in homotypic and heterotypic complexes with mitofusin 1 or 2 on the surface of mitochondria. Thus, mitofusin 2 tethers ER to mitochondria, a juxtaposition required for efficient mitochondrial Ca2+ uptake.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
10 September 2014
Nature 456, 605–610 (2008); doi:10.1038/nature07534 In Fig. 1a of this Article, the representative image of a volume-rendered three-dimensional reconstruction of a z-stack of confocal images of endoplasmic-reticulum-targeted yellow fluorescent protein (ER-YFP) in a Mfn2−/− cell expressing MFN2IYFFT and that of a Mfn1−/− cell appear to be duplicated.
References
Ferri, K. F. & Kroemer, G. Organelle-specific initiation of cell death pathways. Nature Cell Biol. 3, E255–E263 (2001)
Rizzuto, R. & Pozzan, T. Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol. Rev. 86, 369–408 (2006)
Rizzuto, R., Simpson, A. W., Brini, M. & Pozzan, T. Rapid changes of mitochondrial Ca2+ revealed by specifically targeted recombinant aequorin. Nature 358, 325–327 (1992)
Rizzuto, R., Brini, M., Murgia, M. & Pozzan, T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science 262, 744–747 (1993)
Rizzuto, R. et al. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science 280, 1763–1766 (1998)
Hajnoczky, G., Robb-Gaspers, L. D., Seitz, M. B. & Thomas, A. P. Decoding of cytosolic calcium oscillations in the mitochondria. Cell 82, 415–424 (1995)
Scorrano, L. et al. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science 300, 135–139 (2003)
Vance, J. E. Phospholipid synthesis in a membrane fraction associated with mitochondria. J. Biol. Chem. 265, 7248–7256 (1990)
Soltys, B. J. & Gupta, R. S. Interrelationships of endoplasmic reticulum, mitochondria, intermediate filaments, and microtubules–a quadruple fluorescence labeling study. Biochem. Cell Biol. 70, 1174–1186 (1992)
Simmen, T. et al. PACS-2 controls endoplasmic reticulum-mitochondria communication and Bid-mediated apoptosis. EMBO J. 24, 717–729 (2005)
Pitts, K. R., Yoon, Y., Krueger, E. W. & McNiven, M. A. The dynamin-like protein DLP1 is essential for normal distribution and morphology of the endoplasmic reticulum and mitochondria in mammalian cells. Mol. Biol. Cell 10, 4403–4417 (1999)
Szabadkai, G. et al. Drp-1-dependent division of the mitochondrial network blocks intraorganellar Ca2+ waves and protects against Ca2+-mediated apoptosis. Mol. Cell 16, 59–68 (2004)
Cipolat, S., de Brito, O. M., Dal Zilio, B. & Scorrano, L. OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc. Natl Acad. Sci. USA 101, 15927–15932 (2004)
Frezza, C. et al. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell 126, 177–189 (2006)
Chen, H. et al. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J. Cell Biol. 160, 189–200 (2003)
Koshiba, T. et al. Structural basis of mitochondrial tethering by mitofusin complexes. Science 305, 858–862 (2004)
Ishihara, N., Eura, Y. & Mihara, K. Mitofusin 1 and 2 play distinct roles in mitochondrial fusion reactions via GTPase activity. J. Cell Sci. 117, 6535–6546 (2004)
Zuchner, S. et al. Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot–Marie–Tooth neuropathy type 2A. Nature Genet. 36, 449–451 (2004)
Celli, J. et al. Brucella evades macrophage killing via VirB-dependent sustained interactions with the endoplasmic reticulum. J. Exp. Med. 198, 545–556 (2003)
Koch, A., Yoon, Y., Bonekamp, N. A., McNiven, M. A. & Schrader, M. A role for Fis1 in both mitochondrial and peroxisomal fission in mammalian cells. Mol. Biol. Cell 16, 5077–5086 (2005)
Chen, K. H. et al. Dysregulation of HSG triggers vascular proliferative disorders. Nature Cell Biol. 6, 872–883 (2004)
Marsh, B. J., Mastronarde, D. N., Buttle, K. F., Howell, K. E. & McIntosh, J. R. Organellar relationships in the Golgi region of the pancreatic beta cell line, HIT-T15, visualized by high resolution electron tomography. Proc. Natl Acad. Sci. USA 98, 2399–2406 (2001)
Manders, E. M., Verbeek, F. J. & Aten, J. A. Measurement of co-localisation of objects in dual-colour confocal images. J. Microsc. 169, 375–382 (1993)
Annis, M. G. et al. Endoplasmic reticulum localized Bcl-2 prevents apoptosis when redistribution of cytochrome c is a late event. Oncogene 20, 1939–1952 (2001)
Rojo, M., Legros, F., Chateau, D. & Lombes, A. Membrane topology and mitochondrial targeting of mitofusins, ubiquitous mammalian homologs of the transmembrane GTPase Fzo. J. Cell Sci. 115, 1663–1674 (2002)
Chen, H., Chomyn, A. & Chan, D. C. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J. Biol. Chem. 280, 26185–26192 (2005)
Karbowski, M., Norris, K. L., Cleland, M. M., Jeong, S. Y. & Youle, R. J. Role of Bax and Bak in mitochondrial morphogenesis. Nature 443, 658–662 (2006)
Csordas, G. et al. Structural and functional features and significance of the physical linkage between ER and mitochondria. J. Cell Biol. 174, 915–921 (2006)
Pinton, P. et al. The Ca2+ concentration of the endoplasmic reticulum is a key determinant of ceramide-induced apoptosis: significance for the molecular mechanism of Bcl-2 action. EMBO J. 20, 2690–2701 (2001)
Voeltz, G. K., Prinz, W. A., Shibata, Y., Rist, J. M. & Rapoport, T. A. A class of membrane proteins shaping the tubular endoplasmic reticulum. Cell 124, 573–586 (2006)
Mironov, S. L. & Symonchuk, N. ER vesicles and mitochondria move and communicate at synapses. J. Cell Sci. 119, 4926–4934 (2006)
Ishii, K., Hirose, K. & Iino, M. Ca2+ shuttling between endoplasmic reticulum and mitochondria underlying Ca2+ oscillations. EMBO Rep. 7, 390–396 (2006)
Guo, X. et al. Mitofusin 2 triggers vascular smooth muscle cell apoptosis via mitochondrial death pathway. Circ. Res. 101, 1113–1122 (2007)
Pan, X. et al. Nucleus-vacuole junctions in Saccharomyces cerevisiae are formed through the direct interaction of Vac8p with Nvj1p. Mol. Biol. Cell 11, 2445–2457 (2000)
Frezza, C., Cipolat, S. & Scorrano, L. Organelle isolation: functional mitochondria from mouse liver, muscle and cultured fibroblasts. Nature Protocols 2, 287–295 (2007)
Acknowledgements
O.M.d.B. received a ‘Bolsa de Doutoramento’ of FCT Portugal. L.S. is senior scientist of the Dulbecco-Telethon Institute and EMBO YIP. This work was supported by Telethon Italy, Compagnia di San Paolo Italy, United Mitochondrial Disease Foundation USA, Muscular Distrophy Association USA and Swiss National Science Foundation 3100A0-118171.
Author Contributions O.M.d.B. and L.S. conceived and designed the experiments and wrote the manuscript. O.M.d.B. performed all the experiments.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-12, Supplementary Methods and Supplementary Notes. (PDF 8474 kb)
Supplementary Movie 1
Supplementary Movie 1. ER-mitochondria interaction in wt MEF. 180° rotation along the y-axis of a 3D-reconstruction of a z-axis stack of a wt MEF expressing erYFP (green) and mtRFP (red). (MOV 228 kb)
Supplementary Movie 2
Supplementary Movie 2. ER-mitochondria interaction in Mfn2-/- MEF. 180° rotation along the y-axis of a 3D-reconstruction of a z-axis stack of a Mfn2-/- MEF expressing erYFP (green) and mtRFP (red). (MOV 172 kb)
Supplementary Movie 3
Supplementary Movie 3. Electron tomography showing ER-mitochondria interaction in a wt MEF. Rotation along the y and x-axis of a 3D-rendered reconstruction of representative area from an electron tomogram of a wt MEF. Orange objects represent mitochondria, cyan ones cisternae of ER. (MOV 886 kb)
Supplementary Movie 4
Supplementary Movie 4. Electron tomography showing ER-mitochondria interaction in a Mfn2-/- MEF. Rotation along the y and x-axis of a 3D-rendered reconstruction of representative area from an electron tomogram of Mfn2-/- MEF. Orange objects represent mitochondria, cyan ones cisternae of ER. (MOV 815 kb)
Rights and permissions
About this article
Cite this article
de Brito, O., Scorrano, L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 456, 605–610 (2008). https://doi.org/10.1038/nature07534
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature07534
This article is cited by
-
Proteomic analysis of mitochondria associated membranes in renal ischemic reperfusion injury
Journal of Translational Medicine (2024)
-
Combined Effects of Cadmium and Lead on Growth Performance and Kidney Function in Broiler Chicken
Biological Trace Element Research (2024)
-
Mitochondrial dysfunction in metabolism and ageing: shared mechanisms and outcomes?
Biogerontology (2018)
-
Reduced mitochondrial Ca2+ transients stimulate autophagy in human fibroblasts carrying the 13514A>G mutation of the ND5 subunit of NADH dehydrogenase
Cell Death & Differentiation (2016)
-
Transcriptional profiling revealed the anti-proliferative effect of MFN2 deficiency and identified risk factors in lung adenocarcinoma
Tumor Biology (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.