Abstract
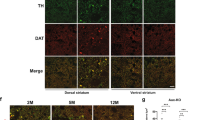
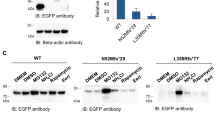
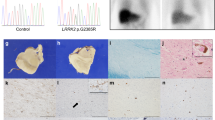
Autosomal recessive juvenile parkinsonism (AR–JP), one of the most common familial forms of Parkinson disease, is characterized by selective dopaminergic neural cell death and the absence of the Lewy body, a cytoplasmic inclusion body consisting of aggregates of abnormally accumulated proteins1. We previously cloned PARK2, mutations of which cause AR–JP (ref. 2), but the function of the gene product, parkin, remains unknown. We report here that parkin is involved in protein degradation as a ubiquitin-protein ligase collaborating with the ubiquitin-conjugating enzyme UbcH7, and that mutant parkins from AR–JP patients show loss of the ubiquitin-protein ligase activity. Our findings indicate that accumulation of proteins that have yet to be identified causes a selective neural cell death without formation of Lewy bodies. Our findings should enhance the exploration of the molecular mechanisms of neurodegeneration in Parkinson disease as well as in other neurodegenerative diseases that are characterized by involvement of abnormal protein ubiquitination, including Alzheimer disease, other tauopathies, CAG triplet repeat disorders and amyotrophic lateral sclerosis3,4,5,6,7,8,9,10.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Mizuno, Y., Hattori, N. & Matsumine, H. Neurochemical and neurogenetic correlates of Parkinson's disease. J. Neurochem. 71, 893–902 (1998).
Kitada, T. et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392, 605–608 (1998).
Mayer, R.J., Landon, M. & Lowe, J. Ubiquitin and the molecular pathology of human disease. in Ubiquitin and the Biology of the Cell. (eds Peters, J.-M., Harris, J.R. & Finley, D.) 429–462 (Plenum, New York, 1998).
Alves-Rodrigues, A., Gregori, L. & Figueiredo-Pereira, M.E. Ubiquitin, cellular inclusions and their role in neurodegeneration. Trends Neurosci. 21, 516–520 (1998).
Floyd, J.A. & Hamilton, B.A. Intranuclear inclusions and the ubiquitin-proteasome pathway: digestion of a red herring? Neuron 24, 765–766 (1999).
Pallares-Trujillo, J., Lopez-Soriano, F.J. & Argiles, J.M. The involvement of the ubiquitin system in Alzheimer's disease. Int. J. Mol. Med. 2, 3–15 (1998).
DiFiglia, M. et al. Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science 277, 1990–1993 (1997).
Cummings, C.J. et al. Mutation of the E6-AP ubiquitin ligase reduces nuclear inclusion frequency while accelerating polyglutamine-induced pathology in SCA1 mice. Neuron 24, 879–892 (1999).
Leigh, P.N. et al. Ubiquitin-immunoreactive intraneuronal inclusions in amyotrophic lateral sclerosis. Morphology, distribution, and specificity. Brain 114, 775–788 (1991).
Kuzuhara, S., Mori, H., Izumiyama, N., Yoshimura, M. & Ihara, Y. Lewy bodies are ubiquitinated. A light and electron microscopic immunocytochemical study. Acta. Neuropathol. (Berl) 75, 345–353 (1988).
Hershko, A. & Ciechanover, A. The ubiquitin system. Annu. Rev. Biochem. 67, 425–479 (1998).
Hochstrasser, M. Ubiquitin-dependent protein degradation. Annu. Rev. Genet. 30, 405–439 (1996).
Coux, O., Tanaka, K. & Goldberg, A.L. Structure and functions of the 20S and 26S proteasomes. Annu. Rev. Biochem. 65, 801–847 (1996).
Leroy, E. et al. The ubiquitin pathway in Parkinson's disease. Nature 395, 451–452 (1998).
Polymeropoulos, M.H. et al. Mutation in the α-synuclein gene identified in families with Parkinson's disease. Science 276, 2045–2047 (1997).
Kruger, R. et al. Ala30Pro mutation in the gene encoding α-synuclein in Parkinson's disease. Nature Genet. 18, 106–108 (1998).
Larsen, C.N., Krantz, B.A. & Wilkinson, K.D. Substrate specificity of deubiquitinating enzymes: ubiquitin C-terminal hydrolases. Biochemistry 37, 3358–3368 (1998).
Spillantini, M.G. et al. α-synuclein in Lewy bodies. Nature 388, 839–840 (1997).
Bennett, M.C. et al. Degradation of α-synuclein by proteasome. J. Biol. Chem. 274, 33855–33858 (1999).
Morett, E. & Bork, P. A novel transactivation domain in parkin. Trends Biochem. Sci. 24, 229–231 (1999).
Joazeiro, C.A.P. et al. The tyrosine kinase negative regulator c-Cbl as a RING-type, E2-dependent ubiquitin-protein ligase. Science 286, 309–312 (1999).
Xie, Y. & Varshavsky, A. The E2-E3 interaction in the N-end rule pathway: the RING-H2 finger of E3 is required for the synthesis of multiubiquitin chain. EMBO J. 18, 6832–6844 (1999).
Harper, J.W. & Elledge, S.J. Skipping into the E2F1-destruction pathway. Nature Cell Biol. 1, E5–E7 (1999).
Deshaies, R.J. SCF and Cullin/RING-H2-based ubiquitin-ligases. Annu. Rev. Cell Dev. Biol. 15, 435–467 (1999).
Lorick, K.L. et al. RING fingers mediate ubiquitin-conjugating enzyme (E2)-dependent ubiquitination. Proc. Natl Acad. Sci. USA 96, 11364–11369 (1999).
Moynihan, T.P. et al. The ubiquitin-conjugating enzymes UbcH7 and UbcH8 interact with RING finger/IBR motif-containing domains of HHARI and H7-AP1. J. Biol. Chem. 274, 30963–30968 (1999).
Martinez-Noel, G., Niedenthal, R., Tamura, T. & Harbers, K. A family of structurally related RING finger proteins interacts specifically with the ubiquitin-conjugating enzyme UbcM4. FEBS Lett. 454, 257–261 (1999).
Hattori, N. et al. Point mutations (Thr240Arg and Gln311Stop) in the parkin gene. Biochem. Biophys. Res. Commun. 249, 754–758 (1998).
Rock, K.L. et al. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell 78, 761–771 (1994).
Suzuki, H. et al. IκBα ubiquitination is catalyzed by an SCF-like complex containing Skp1, cullin-1, and two F-box/WD40-repeat proteins, βTrCP1 and βTrCP2. Biochem. Biophys. Res. Commun. 256, 127–132 (1999).
Acknowledgements
We thank E. Melamed for the DNA sample with a mutation in the Ubl domain.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shimura, H., Hattori, N., Kubo, Si. et al. Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat Genet 25, 302–305 (2000). https://doi.org/10.1038/77060
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/77060
This article is cited by
-
Bi-directional regulation of AIMP2 and its splice variant on PARP-1-dependent neuronal cell death; Therapeutic implication for Parkinson's disease
Acta Neuropathologica Communications (2024)
-
Intracellular delivery of Parkin-RING0-based fragments corrects Parkin-induced mitochondrial dysfunction through interaction with SLP-2
Journal of Translational Medicine (2024)
-
Activation of Ca2+ phosphatase Calcineurin regulates Parkin translocation to mitochondria and mitophagy in flies
Cell Death & Differentiation (2024)
-
Pathogenesis of Parkinson’s disease: from hints from monogenic familial PD to biomarkers
Journal of Neural Transmission (2024)
-
Crosstalk between mitochondrial biogenesis and mitophagy to maintain mitochondrial homeostasis
Journal of Biomedical Science (2023)