Abstract
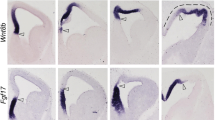
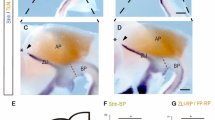
The diencephalon is the primary relay network transmitting sensory information to the anterior forebrain. During development, distinct progenitor domains in the diencephalon give rise to the pretectum (p1), the thalamus and epithalamus (p2), and the prethalamus (p3), respectively. Shh plays a significant role in establishing the progenitor domains. However, the upstream events influencing the expression of Shh are largely unknown. Here, we show that Barhl2 homeobox gene is expressed in the p1 and p2 progenitor domains and the in zona limitans intrathalamica (ZLI) and regulates the acquisition of identity of progenitor cells in the developing diencephalon. Targeted deletion of Barhl2 results in the ablation of Shh expression in the dorsal portion of ZLI and causes thalamic p2 progenitors to take the fate of p1 progenitors and form pretectal neurons. Moreover, loss of Barhl2 leads to the absence of thalamocortical axon projections, the loss of habenular afferents and efferents, and a gross diminution of the pineal gland. Thus, by acting upstream of Shh signaling pathway, Barhl2 plays a crucial role in patterning the progenitor domains and establishing the positional identities of progenitor cells in the diencephalon.




Similar content being viewed by others
References
Sherman SM, Guillery RW, Sherman SM (eds) (2006) Exploring the thalamus and its role in cortical function, 2nd edn. MIT Press, Cambridge, p 484
Puelles L, Rubenstein JL (2003) Forebrain gene expression domains and the evolving prosomeric model. Trends Neurosci 26(9):469–476
Puelles L, Rubenstein JL (1993) Expression patterns of homeobox and other putative regulatory genes in the embryonic mouse forebrain suggest a neuromeric organization. Trends Neurosci 16(11):472–479
Figdor MC, Stern CD (1993) Segmental organization of embryonic diencephalon. Nature 363(6430):630–634
Chatterjee M, Li JY (2012) Patterning and compartment formation in the diencephalon. Front Neurosci 6:66
Kiecker C, Lumsden A (2004) Hedgehog signaling from the ZLI regulates diencephalic regional identity. Nat Neurosci 7(11):1242–1249
Vue TY et al (2009) Sonic hedgehog signaling controls thalamic progenitor identity and nuclei specification in mice. J Neurosci 29(14):4484–4497
Jeong Y et al (2011) Spatial and temporal requirements for sonic hedgehog in the regulation of thalamic interneuron identity. Development 138(3):531–541
Vieira C et al (2005) Thalamic development induced by Shh in the chick embryo. Dev Biol 284(2):351–363
Epstein DJ (2012) Regulation of thalamic development by sonic hedgehog. Front Neurosci 6:57
Scholpp S et al (2006) Hedgehog signalling from the zona limitans intrathalamica orchestrates patterning of the zebrafish diencephalon. Development 133(5):855–864
Ding Q et al (2009) BARHL2 differentially regulates the development of retinal amacrine and ganglion neurons. J Neurosci 29(13):3992–4003
Ding Q et al (2012) BARHL2 transcription factor regulates the ipsilateral/contralateral subtype divergence in postmitotic dI1 neurons of the developing spinal cord. Proc Natl Acad Sci U S A 109(5):1566–1571
Saito T et al (1998) Mammalian BarH homologue is a potential regulator of neural bHLH genes. Dev Biol 199(2):216–225
Suzuki-Hirano A et al (2011) Dynamic spatiotemporal gene expression in embryonic mouse thalamus. J Comp Neurol 519(3):528–543
Vue TY et al (2007) Characterization of progenitor domains in the developing mouse thalamus. J Comp Neurol 505(1):73–91
Pratt T et al (2000) A role for Pax6 in the normal development of dorsal thalamus and its cortical connections. Development 127(23):5167–5178
Peukert D et al (2011) Lhx2 and Lhx9 determine neuronal differentiation and compartition in the caudal forebrain by regulating Wnt signaling. PLoS Biol 9(12):e1001218
Fode C et al (2000) A role for neural determination genes in specifying the dorsoventral identity of telencephalic neurons. Genes Dev 14(1):67–80
Miyashita-Lin EM et al (1999) Early neocortical regionalization in the absence of thalamic innervation. Science 285(5429):906–909
Chen L, Guo Q, Li JY (2009) Transcription factor Gbx2 acts cell-nonautonomously to regulate the formation of lineage-restriction boundaries of the thalamus. Development 136(8):1317–1326
Quina LA et al (2009) Brn3a and Nurr1 mediate a gene regulatory pathway for habenula development. J Neurosci 29(45):14309–14322
Nakagawa Y, Shimogori T (2012) Diversity of thalamic progenitor cells and postmitotic neurons. Eur J Neurosci 35(10):1554–1562
Funato H, Saito-Nakazato Y, Takahashi H (2000) Axonal growth from the habenular nucleus along the neuromere boundary region of the diencephalon is regulated by semaphorin 3F and netrin-1. Mol Cell Neurosci 16(3):206–220
Sahay A et al (2003) Semaphorin 3F is critical for development of limbic system circuitry and is required in neurons for selective CNS axon guidance events. J Neurosci 23(17):6671–6680
Mitchell TN et al (2003) Polymicrogyria and absence of pineal gland due to PAX6 mutation. Ann Neurol 53(5):658–663
Vue TY et al (2013) Thalamic control of neocortical area formation in mice. J Neurosci 33(19):8442–8453
Caballero IM et al (2014) Cell-autonomous repression of Shh by transcription factor Pax6 regulates diencephalic patterning by controlling the central diencephalic organizer. Cell Rep 8(5):1405–1418
Zeltser LM (2005) Shh-dependent formation of the ZLI is opposed by signals from the dorsal diencephalon. Development 132(9):2023–2033
Shimamura K et al (1995) Longitudinal organization of the anterior neural plate and neural tube. Development 121(12):3923–3933
Yao Y et al (2016) Cis-regulatory architecture of a brain signaling center predates the origin of chordates. Nat Genet 48(5):575–580
Juraver-Geslin HA, Gomez-Skarmeta JL, Durand BC (2014) The conserved barH-like homeobox-2 gene barhl2 acts downstream of orthodentricle-2 and together with iroquois-3 in establishment of the caudal forebrain signaling center induced by Sonic Hedgehog. Dev Biol 396(1):107–120
Szabo NE et al (2009) The role of Sonic hedgehog of neural origin in thalamic differentiation in the mouse. J Neurosci 29(8):2453–2466
Acknowledgments
We thank Drs. R. Libby, A. Kiernan, P. White, and the members of Gan laboratory for their insightful discussions and technical assistance. This research was supported by The National Institute of Health grant 1R21EY023104, Zhejiang Province Science Grant 2012C13023-1, and the Research to Prevent Blindness challenge grant to the Department of Ophthalmology at the University of Rochester.
Authors’ Contributions
Q.D. and L.G. designed and conceived the experiments. Q.D., R.B., D.Z., G.L, and L.G. performed the experiments. Q.D., R.B., and L.G wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Qian Ding and Revathi Balasubramanian contributed equally to this work.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Fig. S1
Loss of thalamic nuclei in Barhl2 mutants. PROX1 is expressed in Barhl2 expressing thalamic nuclei at E17.5. Loss of Barhl2 leads to the loss of anterior thalamic nuclei (arrow) (a). Scale bar equals 200 μm (DOC 202 kb)
Fig. S2
Loss of characteristic expression of caudal thalamic neuron markers. A distinct compartmentalization of PAX7 and DBX1 expression in the developing diencephalon is seen at E10.5 and E11.5 (a). This boundary distinction is lost in the Barhl2-null mutant where PAX7 is expressed in the presumptive p2 progenitor domain and the expression of DBX1 is fragmented. At E12.5, Lhx2 and LHX9 are expressed in caudal thalamic p2 progenitors. In Barhl2-null mutants, the expression of both LHX9 (b) and Lhx2 (c) is severely attenuated and is restricted to a thin portion of the mantle zone. Scale bars equal 200 μm (DOC 1010 kb)
Fig. S3
Mis-expression of ASCL1 and SHH in Barhl2-null mutants. DBX1 is expressed in a graded fashion along the p2 progenitor domain, while ASCL1 is expressed in the p1 progenitor domain at E11 (a). Their expression is largely boundary restricted. In the absence of Barhl2, ASCL1 expression extends into the presumptive p2 domain, the graded expression pattern of DBX1 and boundary restriction is lost. There exists a small population of p2 progenitors that express DBX1 and ASCL1. NKX2.2 is expressed in the rostral thalamic p2 and p3 progenitor domain, flanking the SHH expression in ZLI at E11 (c). In Barhl2-null mutants, expression of SHH is caudalized, NKX2.2 expression in the rostral thalamus p2 domain is affected while that in the p3 domain is unaffected. Scale bar equals 200 μm (DOC 915 kb)
Rights and permissions
About this article
Cite this article
Ding, Q., Balasubramanian, R., Zheng, D. et al. Barhl2 Determines the Early Patterning of the Diencephalon by Regulating Shh . Mol Neurobiol 54, 4414–4420 (2017). https://doi.org/10.1007/s12035-016-0001-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0001-5