Abstract
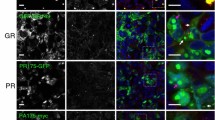
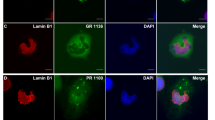
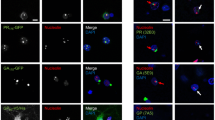
Massive GGGGCC repeat expansion in the first intron of the gene C9orf72 is the most common known cause of familial frontotemporal lobar degeneration (FTLD) and amyotrophic lateral sclerosis (ALS). Despite its intronic localization and lack of an ATG start codon, the repeat region is translated in all three reading frames into aggregating dipeptide-repeat (DPR) proteins, poly-(Gly-Ala), poly-(Gly-Pro) and poly-(Gly-Arg). We took an antibody-based approach to further validate the translation of DPR proteins. To test whether the antisense repeat RNA transcript is also translated, we raised antibodies against the predicted products, poly-(Ala-Pro) and poly-(Pro-Arg). Both antibodies stained p62-positive neuronal cytoplasmic inclusions throughout the cerebellum and hippocampus indicating that not only sense but also antisense strand repeats are translated into DPR proteins in the absence of ATG start codons. Protein products of both strands co-aggregate suggesting concurrent translation of both strands. Moreover, an antibody targeting the putative carboxyl terminus of DPR proteins can detect inclusion pathology in C9orf72 repeat expansion carriers suggesting that the non-ATG translation continues through the entire repeat and beyond. A highly sensitive monoclonal antibody against poly-(Gly-Arg), visualized abundant inclusion pathology in all cortical regions and some inclusions also in motoneurons. Together, our data show that the GGGGCC repeat is bidirectionally translated into five distinct DPR proteins that co-aggregate in the characteristic p62-positive TDP-43 negative inclusions found in FTLD/ALS cases with C9orf72 repeat expansion. Novel monoclonal antibodies against poly-(Gly-Arg) will facilitate pathological diagnosis of C9orf72 FTLD/ALS.






Similar content being viewed by others
References
Al-Sarraj S, King A, Troakes C, Smith B, Maekawa S, Bodi I, Rogelj B, Al-Chalabi A, Hortobagyi T, Shaw CE (2011) p62 positive, TDP-43 negative, neuronal cytoplasmic and intranuclear inclusions in the cerebellum and hippocampus define the pathology of C9orf72-linked FTLD and MND/ALS. Acta Neuropathol 122(6):691–702. doi:10.1007/s00401-011-0911-2
Ash PE, Bieniek KF, Gendron TF, Caulfield T, Lin WL, Dejesus-Hernandez M, van Blitterswijk MM, Jansen-West K, Paul JW 3rd, Rademakers R, Boylan KB, Dickson DW, Petrucelli L (2013) Unconventional translation of C9ORF72 GGGGCC expansion generates insoluble polypeptides specific to c9FTD/ALS. Neuron. doi:10.1016/j.neuron.2013.02.004
Beck J, Poulter M, Hensman D, Rohrer JD, Mahoney CJ, Adamson G, Campbell T, Uphill J, Borg A, Fratta P, Orrell RW, Malaspina A, Rowe J, Brown J, Hodges J, Sidle K, Polke JM, Houlden H, Schott JM, Fox NC, Rossor MN, Tabrizi SJ, Isaacs AM, Hardy J, Warren JD, Collinge J, Mead S (2013) Large C9orf72 hexanucleotide repeat expansions are seen in multiple neurodegenerative syndromes and are more frequent than expected in the UK population. Am J Hum Genet. doi:10.1016/j.ajhg.2013.01.011
Bigio EH, Weintraub S, Rademakers R, Baker M, Ahmadian SS, Rademaker A, Weitner BB, Mao Q, Lee KH, Mishra M, Ganti RA, Mesulam MM (2012) Frontotemporal lobar degeneration with TDP-43 proteinopathy and chromosome 9p repeat expansion in C9ORF72: clinicopathologic correlation. Neuropathology. doi:10.1111/j.1440-1789.2012.01332.x
Boxer AL, Mackenzie IR, Boeve BF, Baker M, Seeley WW, Crook R, Feldman H, Hsiung GY, Rutherford N, Laluz V, Whitwell J, Foti D, McDade E, Molano J, Karydas A, Wojtas A, Goldman J, Mirsky J, Sengdy P, Dearmond S, Miller BL, Rademakers R (2011) Clinical, neuroimaging and neuropathological features of a new chromosome 9p-linked FTD-ALS family. J Neurol Neurosurg Psychiatry 82(2):196–203. doi:10.1136/jnnp.2009.204081
Cruts M, Gijselinck I, Van Langenhove T, van der Zee J, Van Broeckhoven C (2013) Current insights into the C9orf72 repeat expansion diseases of the FTLD/ALS spectrum. Trends Neurosci 36(8):450–459. doi:10.1016/j.tins.2013.04.010
DeJesus-Hernandez M, Mackenzie IR, Boeve BF et al (2011) Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 72(2):245–256. doi:10.1016/j.neuron.2011.09.011
Eisenberg D, Jucker M (2012) The amyloid state of proteins in human diseases. Cell 148(6):1188–1203. doi:10.1016/j.cell.2012.02.022
Fratta P, Poulter M, Lashley T, Rohrer JD, Polke JM, Beck J, Ryan N, Hensman D, Mizielinska S, Waite AJ, Lai MC, Gendron TF, Petrucelli L, Fisher EM, Revesz T, Warren JD, Collinge J, Isaacs AM, Mead S (2013) Homozygosity for the C9orf72 GGGGCC repeat expansion in frontotemporal dementia. Acta Neuropathol. doi:10.1007/s00401-013-1147-0
Gijselinck I, Van Langenhove T, van der Zee J et al (2012) A C9orf72 promoter repeat expansion in a Flanders-Belgian cohort with disorders of the frontotemporal lobar degeneration-amyotrophic lateral sclerosis spectrum: a gene identification study. Lancet Neurol 11(1):54–65. doi:10.1016/S1474-4422(11)70261-7
Gross H, Barth S, Palermo RD, Mamiani A, Hennard C, Zimber-Strobl U, West MJ, Kremmer E, Grasser FA (2010) Asymmetric arginine dimethylation of Epstein-Barr virus nuclear antigen 2 promotes DNA targeting. Virology 397(2):299–310. doi:10.1016/j.virol.2009.11.023
Inukai Y, Nonaka T, Arai T, Yoshida M, Hashizume Y, Beach TG, Buratti E, Baralle FE, Akiyama H, Hisanaga S, Hasegawa M (2008) Abnormal phosphorylation of Ser409/410 of TDP-43 in FTLD-U and ALS. FEBS Lett 582(19):2899–2904. doi:10.1016/j.febslet.2008.07.027
Josephs KA, Hodges JR, Snowden JS, Mackenzie IR, Neumann M, Mann DM, Dickson DW (2011) Neuropathological background of phenotypical variability in frontotemporal dementia. Acta Neuropathol 122(2):137–153. doi:10.1007/s00401-011-0839-6
King A, Al-Sarraj S, Shaw C (2009) Frontotemporal lobar degeneration with ubiquitinated tau-negative inclusions and additional alpha-synuclein pathology but also unusual cerebellar ubiquitinated p62-positive, TDP-43-negative inclusions. Neuropathology 29(4):466–471. doi:10.1111/j.1440-1789.2008.00966.x
Lesage S, Le Ber I, Condroyer C, Broussolle E, Gabelle A, Thobois S, Pasquier F, Mondon K, Dion PA, Rochefort D, Rouleau GA, Durr A, Brice A (2013) C9orf72 repeat expansions are a rare genetic cause of parkinsonism. Brain 136(Pt 2):385–391. doi:10.1093/brain/aws357
Mackenzie IR, Rademakers R, Neumann M (2010) TDP-43 and FUS in amyotrophic lateral sclerosis and frontotemporal dementia. Lancet Neurol 9(10):995–1007. doi:10.1016/S1474-4422(10)70195-2
Mahoney CJ, Beck J, Rohrer JD, Lashley T, Mok K, Shakespeare T, Yeatman T, Warrington EK, Schott JM, Fox NC, Rossor MN, Hardy J, Collinge J, Revesz T, Mead S, Warren JD (2012) Frontotemporal dementia with the C9ORF72 hexanucleotide repeat expansion: clinical, neuroanatomical and neuropathological features. Brain 135(Pt 3):736–750. doi:10.1093/brain/awr361
Mori K, Lammich S, Mackenzie IR, Forne I, Zilow S, Kretzschmar H, Edbauer D, Janssens J, Kleinberger G, Cruts M, Herms J, Neumann M, Van Broeckhoven C, Arzberger T, Haass C (2013) hnRNP A3 binds to GGGGCC repeats and is a constituent of p62-positive/TDP43-negative inclusions in the hippocampus of patients with C9orf72 mutations. Acta Neuropathol. doi:10.1007/s00401-013-1088-7
Mori K, Weng SM, Arzberger T, May S, Rentzsch K, Kremmer E, Schmid B, Kretzschmar HA, Cruts M, Van Broeckhoven C, Haass C, Edbauer D (2013) The C9orf72 GGGGCC repeat is translated into aggregating dipeptide-repeat proteins in FTLD/ALS. Science. doi:10.1126/science.1232927
Neumann M, Kwong LK, Lee EB, Kremmer E, Flatley A, Xu Y, Forman MS, Troost D, Kretzschmar HA, Trojanowski JQ, Lee VM (2009) Phosphorylation of S409/410 of TDP-43 is a consistent feature in all sporadic and familial forms of TDP-43 proteinopathies. Acta Neuropathol 117(2):137–149. doi:10.1007/s00401-008-0477-9
Peabody DS (1989) Translation initiation at non-AUG triplets in mammalian cells. J Biol Chem 264(9):5031–5035
Pearson CE (2011) Repeat associated non-ATG translation initiation: one DNA, two transcripts, seven reading frames, potentially nine toxic entities! PLoS Genet 7(3):e1002018. doi:10.1371/journal.pgen.1002018
Pikkarainen M, Hartikainen P, Alafuzoff I (2010) Ubiquitinated p62-positive, TDP-43-negative inclusions in cerebellum in frontotemporal lobar degeneration with TAR DNA binding protein 43. Neuropathology 30(2):197–199. doi:10.1111/j.1440-1789.2009.01043.x
Rademakers R, Neumann M, Mackenzie IR (2012) Advances in understanding the molecular basis of frontotemporal dementia. Nat Rev Neurol 8(8):423–434. doi:10.1038/nrneurol.2012.117
Renton AE, Majounie E, Waite A et al (2011) A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 72(2):257–268. doi:10.1016/j.neuron.2011.09.010
Todd PK, Oh SY, Krans A, He F, Sellier C, Frazer M, Renoux AJ, Chen KC, Scaglione KM, Basrur V, Elenitoba-Johnson K, Vonsattel JP, Louis ED, Sutton MA, Taylor JP, Mills RE, Charlet-Berguerand N, Paulson HL (2013) CGG repeat-associated translation mediates neurodegeneration in fragile X tremor ataxia syndrome. Neuron 78(3):440–455. doi:10.1016/j.neuron.2013.03.026
van Blitterswijk M, Dejesus-Hernandez M, Niemantsverdriet E, Murray ME, Heckman MG, Diehl NN, Brown PH, Baker MC, Finch NA, Bauer PO, Serrano G, Beach TG, Josephs KA, Knopman DS, Petersen RC, Boeve BF, Graff-Radford NR, Boylan KB, Petrucelli L, Dickson DW, Rademakers R (2013) Association between repeat sizes and clinical and pathological characteristics in carriers of C9ORF72 repeat expansions (Xpansize-72): a cross-sectional cohort study. Lancet Neurol 12(10):978–988. doi:10.1016/S1474-4422(13)70210-2
van der Zee J, Gijselinck I, Dillen L et al (2013) A Pan-European study of the C9orf72 Repeat Associated with FTLD: geographic Prevalence, Genomic Instability and Intermediate Repeats. Hum Mutat 34(2):363–373. doi:10.1002/humu.22244
van der Zee J, Sleegers K, Van Broeckhoven C (2008) Invited article: the Alzheimer disease-frontotemporal lobar degeneration spectrum. Neurology 71(15):1191–1197. doi:10.1212/01.wnl.0000327523.52537.86
Van Langenhove T, van der Zee J, Van Broeckhoven C (2012) The molecular basis of the frontotemporal lobar degeneration-amyotrophic lateral sclerosis spectrum. Ann Med 44(8):817–828. doi:10.3109/07853890.2012.665471
Whitwell JL, Weigand SD, Boeve BF, Senjem ML, Gunter JL, DeJesus-Hernandez M, Rutherford NJ, Baker M, Knopman DS, Wszolek ZK, Parisi JE, Dickson DW, Petersen RC, Rademakers R, Jack CR Jr, Josephs KA (2012) Neuroimaging signatures of frontotemporal dementia genetics: C9ORF72, tau, progranulin and sporadics. Brain 135(Pt 3):794–806. doi:10.1093/brain/aws001
Xi Z, Zinman L, Moreno D, Schymick J, Liang Y, Sato C, Zheng Y, Ghani M, Dib S, Keith J, Robertson J, Rogaeva E (2013) Hypermethylation of the CpG island near the GC repeat in ALS with a C9orf72 expansion. Am J Hum Genet. doi:10.1016/j.ajhg.2013.04.017
Yan J, Fu X, Ge F, Zhang B, Yao J, Zhang H, Qian J, Tomozawa H, Naiki H, Sawashita J, Mori M, Higuchi K (2007) Cross-seeding and cross-competition in mouse apolipoprotein A-II amyloid fibrils and protein A amyloid fibrils. Am J Pathol 171(1):172–180. doi:10.2353/ajpath.2007.060576
Zu T, Gibbens B, Doty NS, Gomes-Pereira M, Huguet A, Stone MD, Margolis J, Peterson M, Markowski TW, Ingram MA, Nan Z, Forster C, Low WC, Schoser B, Somia NV, Clark HB, Schmechel S, Bitterman PB, Gourdon G, Swanson MS, Moseley M, Ranum LP (2011) Non-ATG-initiated translation directed by microsatellite expansions. Proc Natl Acad Sci USA 108(1):260–265. doi:10.1073/pnas.1013343108
Acknowledgments
We thank T. Bachhuber, J. Banzhaf-Strathmann, D. Orozco, B. Schmid, B. Schwenk for helpful discussion. We thank M. K. Schmidt, B. Kraft, I. Pigur for excellent technical assistance. This project was supported by a grant of the Centres of Excellence in Neurodegeneration Research (CoEN) to MC, CVB, CH and DE and the Competence Network for Neurodegenerative Diseases (KNDD) of the Bundesministerium für Bildung und Forschung (BMBF) to CH. KM was supported by a postdoctoral fellowship from the Alexander von Humboldt Foundation. DE was supported by the Helmholtz Young Investigator program. The research leading to these results has received funding from the European Research Council under the European Union’s Seventh Framework Programme (FP7/2007-2013)/ERC Grant agreement n°321366-Amyloid to CH. We acknowledge the VIB Genetic Service Facility and the Antwerp biobank of the Institute Born-Bunge for brain samples, as well as T. Van Langenhove, S. Engelborghs, P. P. De Deyn, A. Sieben and J.-J. Martin for genetic, clinical and pathological diagnoses and brain sectioning and diagnostic immunohistochemistry. The Antwerp site is supported by the MetLife Foundation Award (to CVB), the Belgian Science Policy Office Interuniversity Attraction Poles program; the Foundation for Alzheimer Research (SAO/FRA); the Medical Foundation Queen Elisabeth; the Flemish Government Methusalem excellence program; the Research Foundation Flanders (FWO) and the University of Antwerp Research Fund. I.G. and J.v.d.Z. receive a postdoctoral fellowship of the FWO.
Author information
Authors and Affiliations
Corresponding authors
Additional information
K. Mori and T. Arzberger contributed equally.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mori, K., Arzberger, T., Grässer, F.A. et al. Bidirectional transcripts of the expanded C9orf72 hexanucleotide repeat are translated into aggregating dipeptide repeat proteins. Acta Neuropathol 126, 881–893 (2013). https://doi.org/10.1007/s00401-013-1189-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-013-1189-3